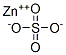
Цинк сернокислый
- английское имяZinc sulphate
- CAS №7733-02-0
- CBNumberCB2727299
- ФормулаZnSO4
- мольный вес161.45
- EINECS231-793-3
- номер MDLMFCD00011302
- файл Mol7733-02-0.mol
| Температура плавления | 100°C |
| Температура кипения | 105°C (estimate) |
| плотность | 1.31 g/mL at 20 °C |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | H2O: soluble |
| форма | Liquid |
| цвет | Colorless |
| РН | 4.0±0.5 |
| Растворимость в воде | Soluble |
| λмакс | λ: 260 nm Amax: <0.02 λ: 280 nm Amax: <0.02 |
| Мерк | 14,10159 |
| LogP | -1.031 (est) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | ZINC SULFATE, ANHYDROUS |
| Справочник по базе данных CAS | 7733-02-0(CAS DataBase Reference) |
| FDA UNII | 0J6Z13X3WO |
| Справочник по химии NIST | Zinc sulfate(7733-02-0) |
| Система регистрации веществ EPA | Zinc sulfate (7733-02-0) |
| UNSPSC Code | 12352302 |
| NACRES | NA.25 |
| Коды опасности | Xn,N,Xi |
| Заявления о рисках | 52/53-50/53-41-22-51/53 |
| Заявления о безопасности | 61-39-26-60 |
| РИДАДР | UN 3082 9/PG 3 |
| WGK Германия | 3 |
| RTECS | ZH5260000 |
| TSCA | Yes |
| кода HS | 2833 29 20 |
| Класс опасности | 9 |
| Группа упаковки | III |
| Банк данных об опасных веществах | 7733-02-0(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H318:При попадании в глаза вызывает необратимые последствия.
H411:Токсично для водных организмов с долгосрочными последствиями.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Цинк сернокислый химические свойства, назначение, производство
Химические свойства
used in zinc plating and as a mordant [KIR84]Физические свойства
The anhydrous sulfate is a colorless rhombohedral crystalline solid; refractive index 1.658; density 3.54 g/cm3; decomposes at 600°C; soluble in water, methanol, and glycerol.The heptahydrate, ZnSO4?7H2O, is a colorless crystalline solid; metallic taste; rhombohedral crystals; effloresces; refractive index 1.457; density 1.957 g/cm3 at 25°C; melts at 100°C; loses all its water molecules at 280°C; decomposes above 500°C; very soluble in water, 96.5 g/100mL at 20°C; soluble in glycerol, 40 g/100 mL; insoluble in alcohol
The hexahydrate, ZnSO4?6H2O constitutes colorless monoclinic or tetragonal crystals; density 2.072 g/cm3at 15°C; loses five water molecules at 70°C; soluble in water.
Использование
The principal commercial preparation of zinc sulfate is the monohydrate granular (36% of zinc) used as a fertilizer. It is also a component of spinning bath in the production of rayon, carbamate fungicides, zinc plating baths, and ophthalmic solutions. Zinc sulfate is used as an accelerating agent in dental impression material, froth flotation agent, and animal feed additive.Методы производства
Zinc sulfate is produced from side streams of electrolytic zinc manufacture. The main source of secondary zinc for zinc sulfate production is galvanizer residues. ZnSO4 is available as the mono-, hexa- and heptahydrates with zinc contents of 36%, 24%, and 22%, respectively.Определение
zinc sulphate: A white crystalline water-soluble compound made by heating zinc sulphide ore in air and dissolving out and recrystallizing the sulphate. The common form is the heptahydrate, ZnSO4.7H2O; r.d. 1.9. This loses water above 30°C to give the hexahydrate and more water is lost above 70°C to form the monohydrate. The anhydrous salt forms at 280°C and this decomposes above 500°C. The compound, which was formerly called white vitriol, is used as a mordant and as a styptic (to check bleeding).Общее описание
Anhydrous Zinc sulphate is a colorless crystalline solid. Zinc sulphate is also obtained as a hexahydrate, ZnSO4.6H2O, and as a heptahydrate ZnSO4.7H2O. All forms are soluble in water. All are noncombustible. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Zinc sulphate is used in the production of rayon, as a feed supplement, used to obtaine lectrolyte zinc, in printing textiles and to make lithopone, to impregnate wood and hides,as an additive to spinning baths for production of synthetic silks, in electroplating, and in animal feeds.Реакции воздуха и воды
Water soluble. Efflorescent in air. Aqueous solutions are acidic.Профиль реактивности
Acidic salts, such as Zinc sulphate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.Угроза здоровью
Inhalation of dust causes irritation of nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract. Contact with eyes or skin causes irritation.Сельскохозяйственное использование
White vitriol is another name for zinc sulphate heptahydrate, and is a commonly used zinc salt. It is widely used as a fertilizer for overcoming zinc deficiency.Промышленное использование
Ferro sulfate (FeSO4·7H2O) is a crystalline substance greenish in color, with a specific gravity of 1.899. Ferro sulfate is obtained from various solutions using a vacuum crystallization method. Ferro sulfate has been used as a depressant and co-depressant in the following applications: (a) depression of sphalerite together with cyanide , (b) depression of fine molybdenite also with cyanide, and (c) in copper/lead separation using a method, based on copper depression by cyanide.Профиль безопасности
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Human systemic effects by ingestion: acute pulmonary edema, agranulocytosis, blood pressure decrease, diarrhea and other gastrointestinal changes, hypermotility, increased pulse rate without blood pressure decrease, level changes for metals other than Na/K/Fe/Ca/P/Cl, microcytosis with or without anemia, normocytic anemia. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. An eye irritant. When heated to decomposition it emits toxic fumes of SOx and ZnO. See also SULFATES and ZINC COMPOUNDS.Методы очистки
Crystallise it from aqueous EtOH or dilute H2SO4 below 39o when it forms the heptahydrate, and between 39o and 70o it forms the hexahydrate, and above 70o the monohydrate is stable. The anhydrous salt is obtained from the hydrates by heating at 280o or lower temperatures in a current of dry air. It decomposes to ZnO and SO2 at 767o. The solubility of the heptahydrate in H2O is 5.88% at 0o, 61.92% at 30o, 66.61% at 35o and 70.05% at 39o.Цинк сернокислый запасные части и сырье
Цинк сернокислый поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8615373025980 | China | 895 | 58 | |
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 |