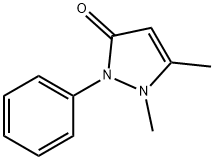
АНТИПИРИН
- английское имяAntipyrine
- CAS №60-80-0
- CBNumberCB8200943
- ФормулаC11H12N2O
- мольный вес188.23
- EINECS200-486-6
- номер MDLMFCD00003146
- файл Mol60-80-0.mol
химическое свойство
| Температура плавления | 109-111 °C(lit.) |
| Температура кипения | 319 °C |
| плотность | 1,19 g/cm3 |
| показатель преломления | 1.5850 (estimate) |
| температура хранения | 2-8°C |
| растворимость | H2O: soluble1 gm in less than 1ml |
| форма | Crystalline Powder |
| пка | pKa 1.4 (Uncertain) |
| цвет | White |
| Биологические источники | rabbit |
| Растворимость в воде | 1000 g/L (20 ºC) |
| Мерк | 14,716 |
| БРН | 157775 |
| Стабильность | Stable. Incompatible with ammonia, strong acids, alkalies, strong oxidizing agents, metallic salts, phenol. |
| LogP | 0.380 |
| Справочник по базе данных CAS | 60-80-0(CAS DataBase Reference) |
| FDA 21 CFR | 310.545; 369.20 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | T3CHA1B51H |
| Код УВД | N02BB01,S02DA03 |
| Справочник по химии NIST | Antipyrine(60-80-0) |
| Система регистрации веществ EPA | Antipyrine (60-80-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.41 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-36/37/38 | |||||||||
| Заявления о безопасности | 26-36-37/39 | |||||||||
| РИДАДР | 3249 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | CD2450000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29331190 | |||||||||
| Токсичность | LD50 orally in rats: 1.8 g/kg (Hart) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
АНТИПИРИН химические свойства, назначение, производство
Химические свойства
Colorless crystal or white crystalline powder. Soluble in benzene, ethanol, water, chloroform, slightly soluble in ether. Odorless, slightly bitter.История
Antipyrine (phenazone) was one of the first important synthetic Analgesic drugs that synthesis in Germany in 1884 by a pupil of Emil Fischer.Использование
Antipyrine has been used for immunoblotting. It has also been used as an internal reference marker for studying the transport characteristics of platinum-containing drug, cisplatin in the human placenta in vitro. Antipyrine is an analgesic and antipyretic that has been given by mouth and as ear drops. It is often used in testing the effects of other drugs or diseases on drug-metabolizing enzymes in the liver. (From Martindale, The Extra Pharmacopoeia, 30th ed, p29)Определение
ChEBI: Antipyrine is a pyrazolone derivative that is 1,2-dihydropyrazol-3-one substituted with methyl groups at N-1 and C-5 and with a phenyl group at N-2. It has a role as a non-narcotic analgesic, an antipyretic, a non-steroidal anti-inflammatory drug, a cyclooxygenase 3 inhibitor, a xenobiotic and an environmental contaminant.Подготовка
Antipyrine (1) was prepared from the reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with methyl iodide in methanol or in acetonitrile containing sodium bicarbonate in water in 58% yield. The reaction of 1-methyl-2-phenylhydrazine (2) with methyl 3-oxobutanoate (3) in acetonitrile gave 1 and pyrazolone 4 . Krohn has been reported the synthesis of 1 from the reaction of 5 with dimethyl sulfate in 71% yield. The reaction of pyrazolone 6 with methanol in the presence of triphenylphosphine afforded 1 (14%) and pyrazole 7 (53%). The same reaction was reported by Pegurier et al by heating the reactants in methanol containing calcium monoxide. Knorr early reported also, the synthesis of antipyrine from heating of 1-phenyl-5-ethoxy-3- methylpyrazole (8) with methyl iodide in methanol.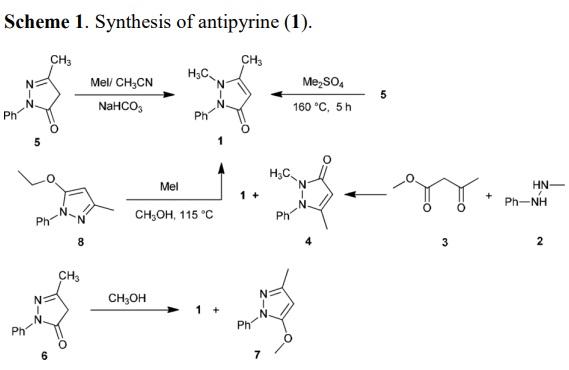
Всемирная организация здравоохранения(ВОЗ)
Phenazone is a pyrazolone derivative chemically related to aminophenazone. Some regulatory authorities have imposed restrictions on its use on these grounds. However, a recent international study showed no statisticallybased evidence of an association with agranulocytosis or aplastic anaemia. Nor does it share with aminophenazone the propensity to produce potentially carcinogenic nitrosamines.Общее описание
Antipyrine is an antipyretic agent used for the symptomatic treatment of acute otitis media, most commonly in combination with benzocaine. One of the earliest widely used analgesics and antipyretics, antipyrine was gradually replaced in common use by other medications including phenacetin (itself later withdrawn because of safety concerns), aspirin, paracetamol and modern NSAIDs such as ibuprofen.Профиль безопасности
A human Doison bv 1 i an unspecified route. Moderately toxic via ingestion, subcutaneous, and intravenous routes. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Методы очистки
Antipyrine crystallises from EtOH/water mixture, *benzene, *benzene/pet ether or hot water (charcoal), and the crystals are dried under a vacuum. [Beilstein 24 H 27, 24 III/IV 75.]АНТИПИРИН запасные части и сырье
сырьё
1of3
запасной предмет
АНТИПИРИН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0533-2091136 +8613864437655 |
China | 100 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-53169958659 +86-13153181156 |
China | 294 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +8619933239880 | China | 861 | 58 | ||
| +86-18239973690 +86-18239973690 |
China | 311 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 |