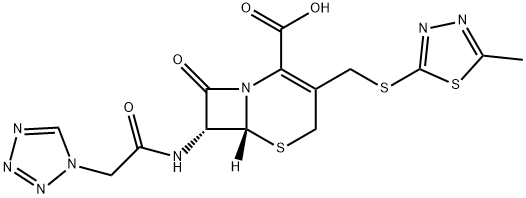
Цефазолин
- английское имяCefazolin
- CAS №25953-19-9
- CBNumberCB3396249
- ФормулаC14H14N8O4S3
- мольный вес454.51
- EINECS247-362-8
- номер MDLMFCD00243010
- файл Mol25953-19-9.mol
химическое свойство
| Температура плавления | 198-200 C |
| плотность | 2.01±0.1 g/cm3(Predicted) |
| температура хранения | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| растворимость | DMSO (Slightly), Methanol (Very Slightly, Heated) |
| пка | pKa 2.15 (Uncertain) |
| форма | Solid |
| цвет | White to Off-White |
| Стабильность | Hygroscopic |
| Справочник по базе данных CAS | 25953-19-9(CAS DataBase Reference) |
| FDA UNII | IHS69L0Y4T |
| Код УВД | J01DB04 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn |
| Заявления о рисках | 20/21/22-36/37/38 |
| Заявления о безопасности | 26-36 |
| WGK Германия | 3 |
| кода HS | 2941906000 |
| Банк данных об опасных веществах | 25953-19-9(Hazardous Substances Data) |
| Токсичность | mouse,LD50,intramuscular,4gm/kg (4000mg/kg),Byoin Yakugaku. Hospital Pharmacology. Vol. 3, Pg. 220, 1978. |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P342+P311:При возникновении симптомов астмы или затрудненного дыхания обратиться за медицинской помощью.
Цефазолин химические свойства, назначение, производство
Описание
Cefazolin has the natural acetyl side chain at C-3 replaced by a thio-linked thiadiazole ring. Although this group is an activating leaving group, the moiety is not subject to the inactivating host hydrolysis reaction that characterizes cephapirin. At C-7, it possesses a tetrazoylmethylene unit. Cefazolin is less irritating on injection than its cohort in this generation of drugs and has a longer half-life than cephapirin. Its dosing should be reduced in the presence of renal impairment. It is comparatively unstable and should be protected from heat and light.Химические свойства
needlesИспользование
Cefazolin is an antibacterial compound derived from 7-amino-cephalosporanic acid.Определение
ChEBI: A cephalosporin compound having [(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl and (1H-tetrazol-1-ylacetyl)amino side-groups.Антимикробная активность
Enterobacter, Klebsiella, Providencia, Serratia spp. and Pr. vulgaris are all resistant. B. fragilis is resistant, but other anaerobes are susceptible.Фармакокине?тика
DistributionThe volume of distribution is the smallest of the cephalosporins in group 1, perhaps an indication of relative confinement to the plasma space. It crosses inflamed synovial membranes, but the levels achieved are well below those of the simultaneous serum levels and entry to the CSF is poor. In patients receiving 10 mg/kg by intravenous bolus, mean concentrations in cancellous bone were 3.0 mg/kg when the mean serum concentration was 33 mg/L, giving a bone:serum ratio of 0.09. Some crosses the placenta, but the concentrations found in the fetus and membranes are low.
Metabolism and excretion
It is not metabolized. Around 60% of the dose is excreted in the urine within the first 6 h, producing concentrations in excess of 1 g/L. Excretion is depressed by probenecid. The renal clearance is around 65 mL/min and declines in renal failure, when the half-life may rise to 40 h, although levels in the urine sufficient to inhibit most urinary pathogens are still found. It is moderately well removed by hemodialysis and less well by peritoneal dialysis.
Levels sufficient to inhibit a number of enteric organisms likely to infect the biliary tract are found in T-tube bile (17–31 mg/L after a 1 g intravenous dose), but this is principally due to the high serum levels of the drug and the total amounts excreted via the bile are small.
Клиническое использование
Cefazolin has been widely used in surgical prophylaxis, especially in biliary tract (because of the moderately high concentrations achieved in bile), orthopedic, cardiac and gynecological surgery.Побочные эффекты
Side effects are those common to other cephalosporins ,including rare bleeding disorders and encephalopathy in patients in whom impaired excretion or direct instillation leads to very high CSF levels. Neutropenia has been described and hypoprothrombinemic bleeding has been attributed to the side chain.Цефазолин запасные части и сырье
Цефазолин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 18627774460 | CHINA | 742 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +8613367258412 | China | 10319 | 58 |