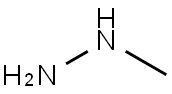
МЕТИЛГИДРАЗИН
- английское имяMethylhydrazine
- CAS №60-34-4
- CBNumberCB7853951
- ФормулаCH6N2
- мольный вес46.07
- EINECS200-471-4
- номер MDLMFCD00007621
- файл Mol60-34-4.mol
| Температура плавления | -21 °C |
| Температура кипения | 88-90 °C(lit.) |
| плотность | 0.875 g/mL at 20 °C(lit.) |
| плотность пара | 1.6 (vs air) |
| давление пара | 37.5 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 70 °F |
| температура хранения | Flammables area |
| растворимость | Soluble in alcohol and ether (Weast, 1986) |
| пка | 8.84±0.70(Predicted) |
| форма | liquid |
| цвет | colorless to pale yellow |
| Пределы взрываемости | 97% |
| Растворимость в воде | soluble |
| Мерк | 13,6109 |
| БРН | 635645 |
| Пределы воздействия | Potential occupational carcinogen. NIOSH REL: 2-h ceiling 0.04 ppm (0.08 mg/m3), IDLH 20 ppm; OSHA PEL: ceiling 0.2 ppm (0.35 mg/m3); ACGIH TLV: TWA 0.01 ppm (adopted). |
| Стабильность | Stable. Flammable. Hygroscopic. Incompatible with strong oxidizing agents, copper, iron and their alloys. |
| Справочник по базе данных CAS | 60-34-4(CAS DataBase Reference) |
| FDA UNII | UWA30B5Z1J |
| Справочник по химии NIST | Hydrazine, methyl-(60-34-4) |
| Система регистрации веществ EPA | Methyl hydrazine (60-34-4) |
| Коды опасности | F,T+,N,T | |||||||||
| Заявления о рисках | 11-24/25-26-34-40-51/53-45-23/24/25-50/53 | |||||||||
| Заявления о безопасности | 16-26-28-36/37/39-45-60-53-24/25-61 | |||||||||
| РИДАДР | UN 1244 6.1/PG 1 | |||||||||
| OEL | Ceiling: 0.04 ppm (0.08 mg/m3) [2-hr] | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | MV5600000 | |||||||||
| Температура самовоспламенения | 385 °F | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 29280000 | |||||||||
| Банк данных об опасных веществах | 60-34-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in mice, rats: 33.0, 32.5 mg/kg (Witkin); orally in rats: 70.7 mg/kg (Gregory) | |||||||||
| ИДЛА | 20 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)





-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H300+H310+H330:Смертельно при проглатывании, при контакте с кожей или при вдыхании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
МЕТИЛГИДРАЗИН химические свойства, назначение, производство
Описание
Methyl hydrazine, CH3NHNH2, is a colorless, hygroscopic liquid with an ammonia-like odor. It is soluble in water, with a specific gravity of 0.87, which is lighter than water. Methyl hydrazine is toxic by inhalation and ingestion, and is a suspected human carcinogen. The TLV ceiling is 0.2 ppm in air, and the IDLH is 50 ppm. The target organs are the central nervous system, respiratory system, liver, blood, eyes, and cardiovascular system. The four-digit UN identification number is 1244. The NFPA 704 designation is health 4, flammability 3, and reactivity 2. The primary uses are as a missile propellant and a solvent.Химические свойства
colourless liquid with an ammonia-like odourФизические свойства
Fuming, clear, colorless liquid with an ammonia-like odor. Odor threshold concentrations ranged from 1 to 3 ppm (quoted, Keith and Walters, 1992).Использование
Methylhydrazine is used in missile propellants and as a solvent and chemical intermediate.Методы производства
Methylhydrazine ignites spontaneously on contact with strong oxidizing agents. It is prepared commercially from the reaction of monochloroamine and monomethylamine.Реакции воздуха и воды
Highly flammable. Often ignites spontaneously. Exposure to air on a large surface may result in spontaneous ignition [Def. Res. and Eng. 27. 1963]. Water soluble. Solutions are highly alkaline and generate heat when water is added.Профиль реактивности
Methylhydrazine is a powerful reducing agent. Ignites upon contact with oxidizing agents i.e. dinitrogen tetraoxide, hydrogen peroxide [Hawley]. Water used to extinguish a fire may cause pollution and should be diked for later disposal. Gives basic solutions with water that generate heat when water is added.Опасность
Flammable, dangerous fire risk, vapors mayexplode, may self-ignite in air and on contact withoxidizing agents. Toxic by ingestion and inhalation.Eye and upper respiratory tract irritant, lung cancerand liver damage. Possible carcinogen.Угроза здоровью
Methyl hydrazine vapors are extremely toxic and the liquid is corrosive to skin. Methyl hydrazine is the strongest convulsant and the most toxic of methyl-substituted hydrazine derivatives. It is more toxic than hydrazine. At high doses, it is a strong central nervous system poison that can lead to convulsions and death. Skin rash may be aggravated by skin exposure.Пожароопасность
Extremely flammable; ignites spontaneously under almost all normal temperature conditions. Water used to extinguish a fire may cause pollution and should be diked for later disposal. Water may be ineffective in extinguishing fires due to the chemical's low flash point. Because of the wide flammability limits, low flash point, and reignition hazard, dry chemicals, carbon dioxide, water spray, and foam may not be as effective as water dilution of fire area. The vapor is heavier than air; thus Methylhydrazine may accumulate sufficiently to flash back. Methylhydrazine fires produce irritating nitrogen oxides. Ignites spontaneously in air when in contact with porous materials (e.g., earth, asbestos, wood, or cloth). Also ignites spontaneously on contact with strong oxidizing agents (e.g., fluorine, chlorine trifluoride, fuming nitric acid, and nitrogen tetroxide). Heat or flame should be avoided because chemical is extremely flammable and explosive.Профиль безопасности
Suspected carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Poison by inhalation, ingestion, skin contact, intraperitoneal, subcutaneous, and intravenous routes. Experimental reproductive effects. Human mutation data reported. Corrosive to skin, eyes and mucous membranes. May self-ignite in air. Very dangerous fire hazard when exposed to heat or flame. To fight fire, use alcohol foam, CO2, dry chemical. Explosive in the form of vapor when exposed to heat or flame. A powerful reducing agent. It is hypergolic with many oxidants (e.g., dinitrogen tetraoxide and hydrogen peroxide). When heated to decomposition it emits toxic fumes of NOx.Возможный контакт
MMH has been used as the propellant in liquid propellant rockets; it is also used as a solvent and as an organic intermediate.Канцерогенность
The carcinogenicity of methylhydrazine has been extensively investigated. In two studies, no compound-related increase in tumor incidence was observed in mice treated orally with methylhydrazine . In other studies, methylhydrazine produced lung tumors in mice and malignant histiocytoma of the liver and cecal tumors in hamsters when administered in drinking water at concentrations of 0.01%. Potential carcinogenicity from vapor exposure to methylhydrazine was also investigated in rats, dogs, hamsters, and mice. Exposures to methylhydrazine at concentrations of 0.02 ppm (rats and mice only) and 0.2, 2, and 5 ppm (rats and hamsters only)were conducted for 6 h/day, 5 days/week, for a year, followed by observation for 1 year.Экологическая судьба
Biological. It was suggested that the rapid disappearance of methylhydrazine in sterile and nonsterile soil (Arrendondo fine sand) under aerobic conditions was due to chemical oxidation. Although the oxidation product was not identified, it biodegraded to carbon dioxide in the nonsterile soil. The oxidation product did not degrade in the sterile soil (Ou and Street, 1988).Перевозки
UN1244 Methylhydrazine, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, 8-Corrosive material, Inhalation Hazard Zone AМетоды очистки
Dry with BaO, then distil it in a vacuum. Store it under nitrogen. [Beilstein 4 IV 3322.]Несовместимости
May form explosive mixture with air. Methyl hydrazine is a highly reactive reducing agent and a medium strong base. May explode if heated. Violent reaction with strong oxidizers, such as fluorine, chlorine, combustibles, nitric acid; hydrogen peroxide. Incompatible with acids, alcohols, glycols, isocyanates, phenols, cresols; porous materials, such as earth, asbestos, wood and cloth. Oxides of iron or copper, manganese, lead, copper or their alloys can lead to fire and explosions. Attacks cork, some plastics, coatings and rubber.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. There are 2 alternatives: Dilute with water, neutralize with sulfuric acid, then flush to sewer with large volumes of water or incinerate with added flammable solvent in furnace equipped with afterburner and alkaline scrubber.МЕТИЛГИДРАЗИН запасные части и сырье
сырьё
1of2
запасной предмет
- 1-метил-3-фенил-1H-пиразол-5-карбальдегид
- ETHYL 1-METHYL-5-PROPYL-1H-PYRAZOLE-3-CARBOXYLATE
- (5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-YL)METHANAMINE
- 1-Methyl-5-propyl-1H-pyrazole-3-carboxylic acid amide ,97%
- 1-METHYL-4-NITRO-3-PROPYL-1H-PYRAZOLE-5-CARBOXYLIC ACID
- 1-МЕТИЛ-5-(ТРИФТОРМЕТИЛ)-1H-ПИРАЗОЛ-3-ОЛ
- 1,5-диметил-1H-пиразол-3-карбоновая кислота
- 5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE
- 1-Methyl-3-propyl-1H-pyrazole-5-carboxamide ,97%
- 5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE-4-CARBONYL CHLORIDE
- 2,2,2-TRIFLUORO-1-(3-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-YL)ETHANONE
- 4-хлор-1,3-диметил-1H-пиразолo[3,4-b]пиридин-5-карбоновая кислота
- 1,4,6-Trimethyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine ,97%
- 1-МЕТИЛ-1H-ПИРАЗОЛО[3,4-B]ПИРИДИН-3-ИЛАМИН
- 1-Метил-3-трифторметил-1Н-пиразол
- ETHYL 2-METHYL-3-(TRIFLUOROMETHYL)PYRAZOLE-4-CARBOXYLATE
- 1-Methyl-3-propyl-1H-pyrazole-5-carbohydrazide ,97%
- 1,5-диметил-1H-пиразол-3-карбонил хлорид
- 5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-AMINE
- 1,3-диметил-1H-тиенo[2,3-c]пиразол-5-карбоновая кислота
- 5-AMINO-3-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
- ЭТИЛОВЫЙ ЭФИР 3-ЭТИЛ-1-МЕТИЛ-1Н-ПИРАЗОЛ-5-КАРБОКСИЛОВОЙ КИСЛОТЫ
- 5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE-4-CARBONITRILE
- 1-МЕТИЛ-3-ПРОПИЛПИРАЗОЛ-5-КАРБОКСИЛОВАЯ КИСЛОТА
- Procarbazine hydrochloride
- 1-METHYL-5-(1H-PYRROL-1-YL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
- 5-хлор-1,3-диметил-1H-пиразол-4-карбальдегид
- ETHYL 1-METHYL-5-(1H-PYRROL-1-YL)-1H-PYRAZOLE-4-CARBOXYLATE
- 4-Бром-1 ,3,5-триметил-1Н-пиразол
- 1,3,5-триметил-1Н-пиразол
1of8