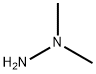
1,1-ДИМЕТИЛГИДРАЗИН
- английское имя1,1-Dimethylhydrazine
- CAS №57-14-7
- CBNumberCB0437549
- ФормулаC2H8N2
- мольный вес60.1
- EINECS200-316-0
- номер MDLMFCD00007628
- файл Mol57-14-7.mol
| Температура плавления | -57.2 °C |
| Температура кипения | 60-62 °C(lit.) |
| плотность | 0.79 g/mL at 20 °C(lit.) |
| плотность пара | 1.94 (vs air) |
| давление пара | 103 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 34 °F |
| температура хранения | 2-8°C |
| растворимость | Miscible with alcohol, N,N-dimethylformamide, ether, hydrocarbons (Windholz et al., 1983), and many other polar solvents. |
| пка | 8.19±0.18(Predicted) |
| форма | Colorless liquid |
| Пределы взрываемости | 95% |
| Растворимость в воде | miscible |
| Чувствительный | Hygroscopic |
| Мерк | 14,3247 |
| БРН | 605261 |
| констант закона Генри | 2.45(x 10-9 atm?m3/mol) at 25 °C (quoted, Mercer et al., 1990) |
| Пределы воздействия | TLV-TWA skin 0.5 ppm (1.0 mg/m3 ) (ACGIH, MSHA, and OSHA); carcinogenicity: Animal Sufficient Evidence (IARC), Suspected Carcinogen (ACGIH). |
| Справочник по базе данных CAS | 57-14-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6 |
| FDA UNII | 4WPQ90N53J |
| Предложение 65 Список | 1,1-Dimethylhydrazine (UDMH) |
| Справочник по химии NIST | Hydrazine, 1,1-dimethyl-(57-14-7) |
| МАИР | 2B (Vol. 4, Sup 7, 71) 1999 |
| Система регистрации веществ EPA | 1,1-Dimethylhydrazine (57-14-7) |
| UNSPSC Code | 41116105 |
| NACRES | NA.22 |
| Коды опасности | F,T,N | |||||||||
| Заявления о рисках | 45-11-23/25-34-51/53-37-23/24/25 | |||||||||
| Заявления о безопасности | 53-45-61-36/37 | |||||||||
| РИДАДР | UN 1163 6.1/PG 1 | |||||||||
| OEL | Ceiling: 0.06 ppm (0.15 mg/m3) [2-hr] | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | MV2450000 | |||||||||
| Температура самовоспламенения | 478 °F | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 29280090 | |||||||||
| Банк данных об опасных веществах | 57-14-7(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for rats 122 mg/kg, mice 265 mg/kg (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 15 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)





-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H301+H311+H331:Токсично при проглатывании, при контакте с кожей или при вдыхании.
H350:Может вызывать раковые заболевания.
H411:Токсично для водных организмов с долгосрочными последствиями.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
1,1-ДИМЕТИЛГИДРАЗИН химические свойства, назначение, производство
Химические свойства
1,1-Dimethylhydrazine is a colorless, flammable, hygroscopic liquid that gradually turns yellow on contact with air and is miscible with water. 1,1-Dimethylhydrazine is primar- ily used as a high-energy fuel in military applications, as a rocket propellant and fuel for thrusters, and small electrical power-generating units. 1,1-Dimethylhydrazine is also used in the manufacture of a plant growth regulator, in chemical synthesis, in photographic chemicals, as a stabilizer for fuel additives, and as an absorbent for acid gases. Exposure to 1,1-dimethylhydrazine usually occurs at the workplace during use and handling of the chemical substance. No information is available on the carcinogenic effects of 1,1-dimethylhydrazine in humans. Carcinogenic effects were observed in mice and rats exposed to 1,1-dimethylhydrazine by inhalation, but the carcinogenicity could not be defi nitively attributed to 1,1-dimethylhydrazine because of the presence of contaminants in the study. The US EPA has not classifi ed 1,1-dimethylhydrazine for potential carcinogenicity, while the IARC has classifi ed 1,1-dimethylhydrazine as Group 2B, meaning a possible human carcinogen.Физические свойства
Clear, colorless fuming liquid with an amine-like odor. Turns yellow on exposure to air. Odor detection threshold concentrations ranged from 6.1 to 14 ppmv (Jacobson et al., 1955).Использование
1,1-Dimethylhydrazine is used in rocket fuel.Определение
ChEBI: A member of the class of hydrazines that is hydrazine substituted by two methyl groups at position 1.Общее описание
A clear colorless liquid with an ammonia-like odor. Flash point 0°F. Corrosive to the skin. Less dense than water and soluble in water. Vapors are heavier than air and very toxic by inhalation, attacking the eyes and respiratory system. Prolonged exposure of containers to heat may result in their violent rupturing and rocketing due to decomposition. Generates toxic oxides of nitrogen when burned. Vapors may travel to a source of ignition and a flame can flashback to the source of vapors. Used as a rocket propellant and to make other chemicals.Реакции воздуха и воды
Highly flammable over a wide range of vapor concentrations. May ignite spontaneously when spread on a large surface exposed to the air. [Def. Res. and Eng., pp 299-300(1963)]. Dissolves and slowly decomposes in water.Профиль реактивности
1,1-Dimethylhydrazine is a powerful reducing agent. Ignition can occur on contact with oxidizing agents such hydrogen peroxide and fuming nitric acid, [Haz. Chem. Data(1966)]. Also reacts as a base to neutralize acids in exothermic reactions.Угроза здоровью
1,1-Dimethylhydrazine exhibits high acute toxicity as a result of exposure by all routes. Death or permanent injury may result after very short exposure to small quantities. Chronic exposure may cause pneumonia, liver damage, and kidney damage.Пожароопасность
Vapor may explode if ignited in an enclosed area. Vapors may travel to a source of ignition and flashback. Runoff to sewer may create fire or explosion hazard. When 1,1-Dimethylhydrazine decomposes, 1,1-Dimethylhydrazine gives off toxic nitrogen compound fumes. Dissolves, swells, and disintegrates many plastics. Dangerous when exposed to heat, flame, or oxidizers. Hazardous polymerization may not occur.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and teratogenic data. Other experimental reproductive effects. Poison byingestion, intraperitoneal, intravenous, and intracerebral routes. Moderately toxic by inhalation and skin contact. Human mutation data reported. A plant growth control agent. Corrosive. A powerful reducing agent. A dangerous fire hazard. It is hypergolic with many oxidants (e.g., dinitrogen tetroxide, hydrogen peroxide, and nitric acid). Dangerous when exposed to heat, flame, or oxidizers; can react vigorously with oxidning materials such as air, fuming HNO3, (HNO3 + N2O4), NO. A high-energy propellant for liquid-fueled rockets. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of NOx. See also HYDRAZINE.Канцерогенность
1,1-Dimethylhydrazine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.Экологическая судьба
Chemical/Physical. Releases toxic nitrogen oxides when heated to decomposition (Sax and Lewis, 1987). Ignites spontaneously in air or in contact with hydrogen peroxide, nitric acid, or other oxidizers (Patnaik, 1992).N-Nitrosodimethylamine was the major product of ozonation of 1,1-dimethylhydrazine in the dark. Hydrogen peroxide, methyl hydroperoxide, and methyl diazene were also identified (HSDB, 1989).
Методы очистки
Fractionally distil the hydrazine through a 4-ft column packed with glass helices. Precipitate it as its oxalate from diethyl ether solution. After crystallising from 95% EtOH, the salt is decomposed with aqueous saturated NaOH, and the free base is distilled, dried over BaO and redistilled [McBride & Kruse J Am Chem Soc 79 572 1957]. Distillation and storage should be under nitrogen. [Beilstein 4 IV 3322.]1,1-ДИМЕТИЛГИДРАЗИН запасные части и сырье
сырьё
1of2
запасной предмет
1of2