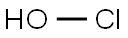
hypochlorous acid
- русский язык имя
- английское имяhypochlorous acid
- CAS №7790-92-3
- CBNumberCB8922249
- ФормулаClHO
- мольный вес52.46
- EINECS232-232-5
- номер MDLMFCD00871734
- файл Mol7790-92-3.mol
химическое свойство
| Температура кипения | 125-130 °C(Press: 11 Torr) |
| плотность | 1.1655 g/cm3 |
| растворимость | soluble in H2O |
| пка | pK (25°) 7.49 |
| форма | stable only in solution |
| цвет | green-yellow; stable only in aqueous solution |
| LogP | 0.710 (est) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | HYPOCHLOROUS ACID |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | 712K4CDC10 |
| Система регистрации веществ EPA | Hypochlorous acid (7790-92-3) |
hypochlorous acid химические свойства, назначение, производство
Описание
Hypochlorous acid [7790-92-3], HOCl, Mr 52.5, is only moderately stable in aqueous solution. It is colorless when dilute and yellowish at higher concentrations. Hypochlorous acid is one of the most powerful oxidizing agents known. Hypochlorous acid solution decomposes exothermically. The main decomposition products are hydrochloric acid and oxygen.Химические свойства
Greenish-yellow aqueous solution. Highly unstable, weak acid. Decomposes to hydrogen chloride and oxygen. Can exist only in dilute solutions.Физические свойства
Greenish-yellow aqueous solution; unstable; weak acid, pKa7.40 at 25°C;soluble in water.Использование
Disinfectant. Because of its instability, hypochlorous acid is not used extensively as an oxidizing or bleaching agent. It was used mainly in the water treatment industry for slime control, for treatment of drinking water, and for sterilization of swimming pools. These applications have now been taken over by the more stable hypochlorites.Определение
ChEBI: Hypochlorous acid is a chlorine oxoacid with formula HOCl; a weak, unstable acid, it is the active form of chlorine in water. It has a role as a human metabolite, an EC 3.1.1.7 (acetylcholinesterase) inhibitor and an EC 2.5.1.18 (glutathione transferase) inhibitor. It is a member of reactive oxygen species and a chlorine oxoacid. It is a conjugate acid of a hypochlorite.Подготовка
Hypochlorous acid is obtained by dissolving chlorine in water, or by addingbleaching powder or sodium hypochlorite to water. A better method of pro-duction is passing chlorine gas into a well-agitated suspension of mercuricoxide:2Cl2 + 2HgO + H2O → HgO•HgCl2 + 2HOCl
or by distilling chlorine hexahydrate and mercuric oxide at low pressure:
2Cl2•6H2O + HgO → 2HOCl + HgCl2 + 5H2O
The latter process can yield a 25% acid solution.
Hypochlorous acid also may be obtained by hydrolysis of chlorine monoflu-oride:
ClF + H2O → H+ + F¯ + HOCl
The above reaction may be explosive and is not recommended for preparinghypochlorous acid.
Опасность
Irritant to skin and eyes.hypochlorous acid запасные части и сырье
запасной предмет
- Pigment Green 18
- 1,1-ДИМЕТИЛГИДРАЗИН
- Vat Yellow 4
- 19-КАРБОКСИАНДРОСТ-4-ЭН-3,17-ДИОН
- 2-Хлор-1-пропанол
- 5-Chloro-3,6-dihydroxy-5-androstan-17-one 3-acetate
- 2-фуранкарбоновой кислоты
- 5-CHLORO-6 B,19-EPOXY-5A -ANDROTANE-3,17-DIONE
- 2-ХЛОРОЦИКЛОГЕКСАНОЛ
- 1,2-бутен оксид
1of4
hypochlorous acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12459 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20314 | 58 | |
| +86-86-1913198-3935 +8617331935328 |
China | 973 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21670 | 55 | |
| +86-19930503282 | China | 8826 | 58 | |
| 0551-65418671 | China | 34571 | 58 | |
| 02768782018 18771942761 |
CHINA | 992 | 58 | |
| +8615255079626 | China | 23556 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3146 | 58 | |
| +86 +8618733132031 |
CHINA | 1313 | 58 |