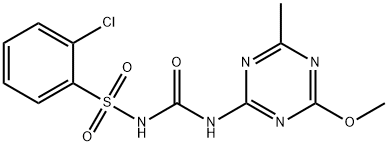
ХЛОРСУЛЬФУРОН
- английское имяChlorsulfuron
- CAS №64902-72-3
- CBNumberCB7486566
- ФормулаC12H12ClN5O4S
- мольный вес357.77
- EINECS265-268-5
- номер MDLMFCD00128059
- файл Mol64902-72-3.mol
химическое свойство
| Температура плавления | 174-178°C |
| плотность | 1.6111 (rough estimate) |
| показатель преломления | 1.5630 (estimate) |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| форма | Solid |
| пка | 4.21±0.10(Predicted) |
| цвет | White to off-white |
| Мерк | 13,2210 |
| БРН | 577255 |
| Справочник по базе данных CAS | 64902-72-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | O6S620ML45 |
| Справочник по химии NIST | Chlorsulfuron(64902-72-3) |
| Пестициды Закон о свободе информации (FOIA) | Chlorsulfuron |
| Система регистрации веществ EPA | Chlorsulfuron (64902-72-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | N,Xi |
| Заявления о рисках | 50/53 |
| Заявления о безопасности | 60-61-36-26 |
| РИДАДР | UN3077 9/PG 3 |
| WGK Германия | 2 |
| RTECS | YS6640000 |
| Банк данных об опасных веществах | 64902-72-3(Hazardous Substances Data) |
| Токсичность | LD50 in male, female rats (mg/kg): 5545, 6293 orally (Levitt) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ХЛОРСУЛЬФУРОН химические свойства, назначение, производство
Описание
Chlorsulfuron is one of the first sulfonylurea herbicides developed and commercialized by DuPont. Dr George Levitt and his team at DuPont first synthesized chlorsulfuron in 1976, and it was commercialized for use as a herbicide in 1981. It is currently registered by DuPont in the United States, Canada, the European Union, Russia, the Ukraine, Australia, New Zealand, South Africa, Saudi Arabia, and in several countries of South America.Compared with many other herbicides that are applied at levels of pounds per acre (or kilograms per acre), sulfonylureas are highly effective at use rates of less than an ounce per acre (approximately 6 g per acre for chlorsulfuron).
Химические свойства
Colorless, odorless crystalsИспользование
Chlorsulfuron is used as a postemergence herbicide for the control of dicotyledonous weeds, with excellent safety for wheat and other cereals crops. While chlorsulfuron is primarily used to control weeds in cereals, it can also be used in range and pasture applications. It is currently only used to a minor extent for nonfood industrial applications and right-of-way purposes.Общее описание
Colorless crystals. Non corrosive. Insoluble in water. Used as an herbicide.Реакции воздуха и воды
Insoluble in water. Reacts slowly with water. The reaction is promoted by acid such that the pH is less than 5.0 (1/2 life of 24-48 hrs.). Reaction is also promoted by polar organic solvents such as methanol and acetone.Сельскохозяйственное использование
Herbicide: A selective systemic herbicide used to control most broadleaf weeds and some annual grasses in wheat, barley, oats, duram, rye, triticale and flax. Applied to non-crop sites such as rights-of-way, fence rows and roadsides.Торговое название
DPX 4189®; FINESSE®; GLEAN®; GLEAN 20DF®; LANDMARK® MP; LASHER®; RIVERDALE CORSAIR®; TELAR® DFВозможный контакт
A selective systemic sulfonylurea herbicide used to control most broadleaf weeds and some annual grasses in wheat, barley, oats, duram, rye, triticale, and flax. Applied to noncrop sites such as rights-of-way, fence rows, and roadsides.Экологическая судьба
Soil. Degrades in soil via hydrolysis followed by microbial degradation forming low molecular weight, inactive compounds. The estimated half-life was reported to range from 4 to 6 weeks (Hartley and Kidd, 1987; Cremlyn, 1991). Microorganisms capable of degrading chlorsulfuron are Aspergillis niger, Streptomyces griseolus and Penicillium sp. (Humburg et al., 1989). One transformation product reported in field soils is 2-chlorobenzenesulfonamide (Smith, 1988)The reported dissipation rate of chlorsulfuron in surface soil is 0.024/day (Walker and Brown, 1983). The persistence of chlorsulfuron decreased when soil temperature and moisture were increased (Walker and Brown, 1983; Thirunarayanan et al., 1985)
Plant. Chlorsulfuron is metabolized by plants to hydroxylated, nonphytotoxic compounds including 2-chloro-N-(((4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino)carbonyl)benzenesulfonamide (Duke et al., 1991). Devine and Born (1985) and Peterson and
Photolytic. The reported photolysis half-lives of chlorsulfuron in distilled water, methanol and natural creek water at λ >290 nm were 18, 92 and 18 hours, respectively. In all cases, 2-chlorobenzene sulfonamide, 2-methoxy-4-methyl-6-amino-1,3,5-triazine and trace amounts of the tentatively identified compound nitroso-2-chlorophenylsulfone formed as photoproducts (Herrmann et al., 1985).
Метаболический путь
Chlorsulfuron is metabolized in wheat and in tolerant broadleaves via different pathways where hydroxylation occurs on the methyl group of the triazine ring and at the phenyl ring of the chlorsulfuron in respective plants. With chemical degradation of chlorsulfuron on dry minerals (Syst.), two pathways of degradation are observed, one of which is directПеревозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Несовместимости
Slowly hydrolyzes in water, releasing ammonia and forming acetate salts. May bencompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Утилизация отходов
It is the responsibility of chemical waste generators to determine the toxicity and physical properties and of a discarded chemical and to properly identify its classification and certification as a hazardous waste and to determine the disposal method. United States Environmental Protection Agency guidelines for the classification determination are listed in 40 CFR Parts 261.3. In addition, waste generators must consult and follow all regional, national, state, and local hazardous waste laws to ensure complete and accurate classification and disposal methods. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulationsХЛОРСУЛЬФУРОН запасные части и сырье
сырьё
1of6
запасной предмет