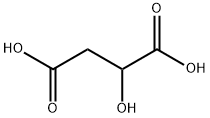
DL-яблочная кислота
- английское имяMalic acid
- CAS №6915-15-7
- CBNumberCB7220795
- ФормулаC4H6O5
- мольный вес134.09
- EINECS230-022-8
- номер MDLMFCD00064212
- файл Mol6915-15-7.mol
химическое свойство
| Температура плавления | 131-133 °C (lit.) |
| Температура кипения | 167.16°C (rough estimate) |
| альфа | [α]D20 -0.5~+0.5° (c=5, H2O) |
| плотность | 1.609 |
| Плотность накопления | 800kg/m3 |
| плотность пара | 4.6 (vs air) |
| давление пара | <0.1 mm Hg ( 20 °C) |
| показатель преломления | 1.3920 (estimate) |
| Fp | 203 °C |
| температура хранения | 2-8°C |
| растворимость | methanol: 0.1 g/mL, clear, colorless |
| пка | 3.4(at 25℃) |
| форма | Solid |
| цвет | White to Almost white |
| Запах | at 100.00 %. odorless |
| РН | 3.33(1 mM solution);2.74(10 mM solution);2.21(100 mM solution); |
| Odor Type | odorless |
| оптическая активность | [α]/D 0.10 to +0.10° |
| Биологические источники | synthetic |
| Растворимость в воде | 558 g/L (20 ºC) |
| Мерк | 14,5707 |
| БРН | 1723539 |
| LogP | -6.14 at 20℃ |
| Справочник по базе данных CAS | 6915-15-7(CAS DataBase Reference) |
| FDA 21 CFR | 184.1069; 582.1069 |
| Специальный комитет по веществам GRAS | Malic acid |
| Рейтинг продуктов питания EWG | 1-4 |
| FDA UNII | 817L1N4CKP |
| Справочник по химии NIST | Hydroxybutanedioic acid(6915-15-7) |
| Система регистрации веществ EPA | Malic acid (6915-15-7) |
| UNSPSC Code | 85151701 |
| NACRES | NA.25 |
больше
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 22-37/38-41-36/37/38-42/43-34 | |||||||||
| Заявления о безопасности | 26-39-37/39-36-36/39 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ON7175000 | |||||||||
| Температура самовоспламенения | 644 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29181980 | |||||||||
| Банк данных об опасных веществах | 6915-15-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 3200 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
DL-яблочная кислота химические свойства, назначение, производство
Использование
Malic acid, HOOCCH(OH).CH2COOH, also known as hydroxysuccinic acid, is a colorless solid. It is soluble in water and alcohol. Malic acid exists in two optically active forms and a racemic mixture. It is used in medicine and found in apples and other fruits.The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
Методы производства
Malic acid is manufactured by hydrating maleic and fumaric acids in the presence of suitable catalysts. The malic acid formed is then separated from the equilibrium product mixture.Определение
ChEBI: Malic acid is a 2-hydroxydicarboxylic acid that is succinic acid in which one of the hydrogens attached to a carbon is replaced by a hydroxy group. It has a role as a food acidity regulator and a fundamental metabolite. It is a 2-hydroxydicarboxylic acid and a C4-dicarboxylic acid. It derives from a succinic acid. It is a conjugate acid of a malate(2-) and a malate.Общее описание
The chiral resolution of DL-malic acid by ligand-exchange capillary electrophoresis was studied.Фармацевтические приложения
Malic acid is used in pharmaceutical formulations as a generalpurpose acidulant. It possesses a slight apple flavor and is used as a flavoring agent to mask bitter tastes and provide tartness. Malic acid is also used as an alternative to citric acid in effervescent powders, mouthwashes, and tooth-cleaning tablets.In addition, malic acid has chelating and antioxidant properties. It may be used with butylated hydroxytoluene as a synergist in order to retard oxidation in vegetable oils. In food products it may be used in concentrations up to 420 ppm.
Therapeutically, malic acid has been used topically in combination with benzoic acid and salicylic acid to treat burns, ulcers, and wounds. It has also been used orally and parenterally, either intravenously or intramuscularly, in the treatment of liver disorders, and as a sialagogue.
Механизм действия
Malic acid is absorbed from the gastrointestinal tract from whence it is transported via the portal circulation to the liver. There are a few enzymes that metabolize malic acid. Malic enzyme catalyzes the oxidative decarboxylation of L-malate to pyruvate with concomitant reduction of the cofactor NAD+ (oxidized form of nicotinamide adenine dinucleotide) or NADP+ (oxidized form of nicotinamide adenine dinucleotide phosphate). These reactions require the divalent cations magnesium or manganese. Three isoforms of malic enzyme have been identified in mammals: a cytosolic NADP+-dependent malic enzyme, a mitochondrial NADP+- dependent malic enzyme and a mitochondrial NAD(P)+-dependent malic enzyme. The latter can use either NAD+ or NADP+ as the cofactor but prefers NAD+. Pyruvate formed from malate can itself be metabolized in a number of ways, including metabolism via a number of metabolic steps to glucose. Malate can also be metabolized to oxaloacetate via the citric acid cycle. The mitochondrial malic enzyme, particularly in brain cells may play a key role in the pyruvate recycling pathway, which utilizes dicarboxylic acids and substrates, such as glutamine, to provide pyruvate to maintain the citric acid cycle activity when glucose and lactate are low.Профиль безопасности
A poison by intraperitoneal route. Moderately toxic by ingestion. A skin and severe eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.Безопасность
Malic acid is used in oral, topical, and parenteral pharmaceutical formulations in addition to food products, and is generally regarded as a relatively nontoxic and nonirritant material. However, concentrated solutions may be irritant.LD50 (rat, oral): 1.6 g/kg(3)
LD50 (rat, IP): 0.1 g/kg
хранилище
Malic acid is stable at temperatures up to 150°C. At temperatures above 150°C it begins to lose water very slowly to yield fumaric acid; complete decomposition occurs at about 180°C to give fumaric acid and maleic anhydride.Malic acid is readily degraded by many aerobic and anaerobic microorganisms. Conditions of high humidity and elevated temperatures should be avoided to prevent caking.
The effects of grinding and humidity on malic acid have also been investigated.
The bulk material should be stored in a well-closed container, in a cool, dry place.
Методы очистки
Crystallise the acid from acetone, then from acetone/CCl4, or from ethyl acetate by adding pet ether (b 60-70o). Dry it at 35o under 1mm pressure to avoid formation of the anhydride. [Beilstein 3 IV 1124.]Несовместимости
Malic acid can react with oxidizing materials. Aqueous solutions are mildly corrosive to carbon steels.Регуляторный статус
GRAS listed. Both the racemic mixture and the levorotatory isomer are accepted as food additives in Europe. The DL and L forms are included in the FDA Inactive Ingredients Database (oral preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.DL-яблочная кислота запасные части и сырье
DL-яблочная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13591747876 +86-13591747876 |
China | 299 | 58 | ||
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12815 | 58 | ||
| +86-66697723 +86-17703311139 |
China | 563 | 58 | ||
| +86-13131129325 | China | 5872 | 58 | ||
| +8615531151365 | China | 18137 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20240 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 |