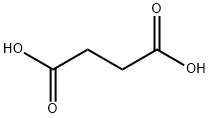
Кислота янтарная
- английское имяSuccinic acid
- CAS №110-15-6
- CBNumberCB9852802
- ФормулаC4H6O4
- мольный вес118.09
- EINECS203-740-4
- номер MDLMFCD00002789
- файл Mol110-15-6.mol
химическое свойство
| Температура плавления | 185 °C |
| Температура кипения | 235 °C |
| плотность | 1.19 g/mL at 25 °C(lit.) |
| Плотность накопления | 940kg/m3 |
| давление пара | 0-0Pa at 25℃ |
| показатель преломления | n |
| FEMA | 4719 | SUCCINIC ACID |
| Fp | >230 °F |
| температура хранения | 2-8°C |
| растворимость | Soluble in ethanol, ethyl ether, acetone and methanol. Insoluble in toluene, benzene, carbon disulfide, carbon tetrachloride and petroleum ether. |
| пка | 4.16(at 25℃) |
| форма | Powder/Solid |
| цвет | White to off-white |
| РН | 3.65(1 mM solution);3.12(10 mM solution);2.61(100 mM solution); |
| Запах | at 100.00 %. wormwood |
| Odor Type | herbal |
| Биологические источники | synthetic |
| Растворимость в воде | 80 g/L (20 ºC) |
| Мерк | 14,8869 |
| БРН | 1754069 |
| Диэлектрическая постоянная | 2.4(26℃) |
| Стабильность | Stable. Substances to be avoided include strong bases, strong oxidizing agents. Combustible. |
| ИнЧИКей | KDYFGRWQOYBRFD-UHFFFAOYSA-N |
| LogP | -0.59 |
| FDA 21 CFR | 184.1091; 582.1091 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SUCCINIC ACID |
| Специальный комитет по веществам GRAS | Succinic acid |
| Справочник по базе данных CAS | 110-15-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | AB6MNQ6J6L |
| Справочник по химии NIST | Butanedioic acid(110-15-6) |
| Система регистрации веществ EPA | Succinic acid (110-15-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.71 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 37/38-41-36/37/38 | |||||||||
| Заявления о безопасности | 26-36/37/39-37/39-39 | |||||||||
| РИДАДР | UN 3265 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WM4900000 | |||||||||
| Температура самовоспламенения | 470 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29171990 | |||||||||
| Банк данных об опасных веществах | 110-15-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2260 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Кислота янтарная химические свойства, назначение, производство
Химические свойства
Succinic acid,C02H(CH2)2C02H, also known as butanedioic acid,butane diacid, and amber acid, is a colorless odorless prisms or white crystalline powder that melts at 185°C (364 of). Soluble in water and alcohol, it is used as a chemical intermediate, Succinic acid is used in lacquers,medicine,dyes,and as a taste modifier.Использование
succinic acid is widely use as organic intermediates for the pharmaceutical, engineering plastics, resins etc.. For the synthesis of sedatives, contraceptives and cancer drugs in the pharmaceutical industry. In the chemical industry for the production of dyes, alkyd resin, glass fiber reinforced plastics, ion exchange resins and pesticides.Определение
ChEBI: An alpha,omega-dicarboxylic acid resulting from the formal oxidation of each of the terminal methyl groups of butane to the corresponding carboxy group. It is an intermediate metabolite in the citric acid cycle.Методы производства
Succinic acid can be produced 1) by oxidation of Butanediol-1,4 with Nitric acid. 2) by oxidation of Tetrahydrofuran with Nitric acid. 3) by hydrogenation of Fumaric acid or Maleic acid.Общее описание
White crystals or shiny white odorless crystalline powder. pH of 0.1 molar solution: 2.7. Very acid taste.Реакции воздуха и воды
Slightly water soluble.Профиль реактивности
Succinic acid reacts exothermically to neutralize bases, both organic and inorganic. Can react with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow in the dry, but systems may absorb water from the air to allow corrosion of iron, steel, and aluminum parts and containers. Reacts slowly with cyanide salts to generate gaseous hydrogen cyanide. Reacts with solutions of cyanides to cause the release of gaseous hydrogen cyanide. May generate flammable and/or toxic gases and heat with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. May react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents. May initiate polymerization reactions.Пожароопасность
Flash point data for Succinic acid are not available. Succinic acid is probably combustible.Профиль безопасности
Moderately toxic by subcutaneous route. A severe eye irritant. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.Канцерогенность
Monosodium succinate was given to groups of 50 male and 50 female Fischer 344 rats in drinking water at levels of 0%, 1%, or 2% for 2 years. No toxic lesion specifically caused by long-term administration of monosodium succinate was detected, and no dose-related increase was found in the incidence of tumors in any organ or tissue. The incidence of C-cell tumors of the thyroid gland of females that received 2% solution was apparently, but not significantly, higher than that in controls. Because C-cell tumors are commonly occurring spontaneous tumors in aging female rats of this strain and the incidence of C-cell tumors in the female control group was lower than that of historical controls for the testing laboratory, the authors concluded that this lesion was not treatment related.Методы очистки
Wash it with diethyl ether. Crystallise it from acetone, distilled water, or tert-butanol. Dry it under vacuum over P2O5 or conc H2SO4. Also purify it by conversion to the disodium salt which, after crystallisation from boiling water (charcoal), is treated with mineral acid to regenerate the succinic acid. The acid is then recrystallised and dried in a vacuum. [Beilstein 2 H 606, 2 IV 1908.]Кислота янтарная запасные части и сырье
сырьё
1of2
запасной предмет
- fatliquor RCF I/II
- Dimethachlon
- 2-Кетоглутаровая кислота
- янтарная кислота, натриевая соль
- N-Бромсукцинимид
- L-аспарагиновой кислоты
- Сукцинимид
- Янтарный ангидрид
- sodium 1,1'-diphosphono propionyloxy phosphonate
- Daminozide
1of4
Кислота янтарная поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +86-0310-8166573 +8618630058311 |
China | 30 | 58 | ||
| +8615175982296 | China | 33 | 58 | ||
| +86-531-88989536 +86-15508631887 |
China | 2956 | 58 | ||
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | ||
| +8613223293093 | China | 80 | 58 | ||
| +86-13131129325 | China | 5872 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 999 | 58 | ||
| +8615531151365 | China | 18137 | 58 |