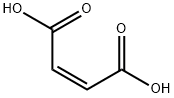
КИСЛОТА малеиновая
- английское имяMaleic acid
- CAS №110-16-7
- CBNumberCB2852803
- ФормулаC4H4O4
- мольный вес116.07
- EINECS203-742-5
- номер MDLMFCD00063177
- файл Mol110-16-7.mol
| Температура плавления | 130-135 °C (lit.) |
| Температура кипения | 275°C |
| плотность | 1.59 g/mL at 25 °C (lit.) |
| Плотность накопления | 750-800kg/m3 |
| давление пара | 0.001Pa at 20℃ |
| показатель преломления | 1.5260 (estimate) |
| Fp | 127 °C |
| температура хранения | Store below +30°C. |
| растворимость | 478.8g/l |
| пка | 1.83(at 25℃) |
| форма | Powder/Solid |
| Удельный вес | 1.59 |
| цвет | White |
| РН | 3.05(1 mM solution);2.21(10 mM solution);1.54(100 mM solution); |
| Запах | Faint odor |
| Растворимость в воде | 790 g/L (25 ºC) |
| Мерк | 14,5703 |
| БРН | 605762 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, bases. |
| ИнЧИКей | VZCYOOQTPOCHFL-OWOJBTEDSA-N |
| LogP | -1.3 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | MALEIC ACID |
| FDA 21 CFR | 175.105; 177.1200 |
| Справочник по базе данных CAS | 110-16-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-3 |
| FDA UNII | 91XW058U2C |
| Справочник по химии NIST | 2-Butenedioic acid (Z)-(110-16-7) |
| Система регистрации веществ EPA | Maleic acid (110-16-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-36/37/38-43 | |||||||||
| Заявления о безопасности | 26-28-37-28A-46-24 | |||||||||
| РИДАДР | 3261 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | OM9625000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29173990 | |||||||||
| Банк данных об опасных веществах | 110-16-7(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H318:При попадании в глаза вызывает необратимые последствия.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H302+H312:Вредно при проглатывании или при попадании на кожу.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
КИСЛОТА малеиновая химические свойства, назначение, производство
Описание
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCHCHCO2H. Maleic acid is the cis-isomer of butenedioic acid, where as fumaric acid is the trans-isomer. It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications.Химические свойства
Maleic acid, also known as maleinic acid and toxilic acid, is a white crystalline (monoclinic) powder and possesses a faint acidulous odor and an astringent taste. It is soluble in water and alcohol. Maleic acid and fumaric acid are the simplest unsaturated carboxylic diacids. These acids experience two-step dissociation in aqueous solutions.They have the same structural formula but different spatial configurations. Fumaric acid is the trans and maleic acid the cis isomer. The physical properties of maleic acid and fumaric acid are very different. The cis isomer is less stable. Maleic acid is used in the preparation of fumaric acid by catalytic isomerization.Физические свойства
Maleic acid is a less stable molecule than fumaric acid. The difference in heat of combustion is 22.7 kJ·mol?1. The heat of combustion is -1355 kJ / mole. Maleic acid is more soluble in water than fumaric acid. The melting point of maleic acid (135 °C) is also much lower than that of fumaric acid (287 °C). Both properties of maleic acid can be explained on account of the intramolecular hydrogen bonding that takes place in maleic acid at the expense of intermolecular interactions, and that are not possible in fumaric acid for geometric reasons.Методы производства
Maleic anhydride is the main source of maleic acid produced by hydration. Maleic anhydride is prepared commercially by the oxidation of benzene or by the reaction of butane with oxygen in the presence of a vanadium catalyst.Определение
ChEBI: Maleic acid is a butenedioic acid in which the double bond has cis- (Z)-configuration. It has a role as a plant metabolite, an algal metabolite and a mouse metabolite. It is a conjugate acid of a maleate(1-) and a maleate.прикладной
Maleic acid is an industrial raw material for the production of glyoxylic acid by ozonolysis. It may be used to form acid addition salts with drugs to make them more stable, such as indacaterol maleate. Maleic acid is also used in manufacturing synthetic resins; in textile processing; in preserving oils and fats; to retard rancidity of fats and oils in 1:10,000 (these are said to keep 3 times longer than those without the acid); dyeing and finishing wool, cotton, and silk; preparing the maleate salts of antihistamines and similar drugs.Реакции
Although not practised commercially, maleic acid can be converted into maleic anhydride by dehydration, to malic acid by hydration, and to succinic acid by hydrogenation (ethanol / palladium on carbon). It reacts with thionyl chloride or phosphorus pentachloride to give the maleic acid chloride (it is not possible to isolate the mono acid chloride). Maleic acid, being electrophilic, participates as a dienophile in many Diels - Alder reactions.Общее описание
Maleic acid is a colorless crystalline solid having a faint odor. Maleic acid is combustible though Maleic acid may take some effort to ignite. Maleic acid is soluble in water. Maleic acid is used to make other chemicals and for dyeing and finishing naturally occurring fibers.Реакции воздуха и воды
Soluble in water.Профиль реактивности
Maleic acid is a colorless to white crystalline solid. Moderately toxic. When heated to decomposition Maleic acid emits irritating fumes and acrid smoke [Lewis, 3rd ed., 1993, p. 790].Опасность
Toxic by ingestion.Угроза здоровью
Inhalation causes irritation of nose and throat. Contact with eyes or skin causes irritation.Пожароопасность
Special Hazards of Combustion Products: Irritating smoke containing maleic anhydride may form in fire.Фармацевтические приложения
Maleic acid is used in the pharmaceutical industry as a pH modifier and a buffering agent.It is also used to prevent rancidity of oils and fats; a ratio of 1 : 10 000 is usually sufficient to retard rancidity. Maleic acid is commonly used as a pharmaceutical intermediate to form the maleate salts of several categories of therapeutic agents, such as salts of antihistamines and other drug substances.Профиль безопасности
Moderately toxic by ingestion and skin contact. Passes through intact skin. A skin and severe eye irritant and a corrosive. Believed to be more toxic than its isomer, fumeric acid. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.Безопасность
Maleic acid is generally regarded as a nontoxic and nonirritant material when used at low levels as an excipient. Maleic acid is used in oral, topical, and parenteral pharmaceutical formulations in addition to food products.LD50 (mouse, oral): 2.40g/kg(7)
LD50 (rabbit, skin): 1.56g/kg
LD50 (rat, oral): 0.708g/kg
Возможный контакт
Maleic acid is used to make artificial resins, antihistamines, and to preserve (retard rancidity) of fats and oilsКанцерогенность
In chronic feeding studies, 12 Osborne–Mendel rats per group were fed 0.5, 1.0, or 1.5% maleic acid in their diets for 2 years. Concentrations of 1.0 and 1.5% maleic acid retarded the growth rate of rats, and all concentrations of maleic acid increased mortality rate; no tumorigenesis was reported. Toxicological differences from controls were not marked, and the pathology was nonspecific.хранилище
Maleic acid converts into the much higher-melting fumaric acid (mp: 287°C) when heated to a temperature slightly above its melting point.Maleic acid is combustible when exposed to heat or flame. The bulk material should be stored in airtight glass containers and protected from light. It is recommended not to store it above 25°C.
Перевозки
UN2215 Maleic acid, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Crystallise the acid from acetone/pet ether (b 60-80o) or hot water. Dry it at 100o. [Beilstein 2 H 748, 2 I 303, 2 II 641, 2 III 1911, 2 IV 2199.]Несовместимости
Maleic acid can react with oxidizing materials. Aqueous solutions are corrosive to carbon steels.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Liquid: incinerate after mixing with a flammable solvent. Use afterburner for complete combustion. Solid: dissolve in a flammable solvent or package in paper and burn. See aboveРегуляторный статус
Included in the FDA Inactive Ingredients Database (IM and IV injections; oral tablets and capsules; topical applications). Included in nonparenteral and parenteral medicines licensed in the UK.КИСЛОТА малеиновая запасные части и сырье
сырьё
запасной предмет
- Фумаронитрил
- Эналаприл
- 2-Phosphonobutane-1,2,4-tricarboxylic acid
- 5-Бром-2-метоксикоричной кислоты
- L-аспарагиновой кислоты
- corrosion inhibitor PBTCA-type
- sodium 1,1'-diphosphono propionyloxy phosphonate
- Винная кислота DL
- Trimebutine maleate
- Polymaleic acid
1of6
КИСЛОТА малеиновая поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined13921770081 | China | 92 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8619933239880 | China | 861 | 58 | ||
| +8613343047651 | China | 3692 | 58 |
КИСЛОТА малеиновая Обзор)
1of4