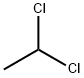
1,1-ДИХЛОРЭТАН
- английское имя1,1-Dichloroethane
- CAS №75-34-3
- CBNumberCB6393704
- ФормулаC2H4Cl2
- мольный вес98.96
- EINECS200-863-5
- номер MDLMFCD00013673
- файл Mol75-34-3.mol
| Температура плавления | 235℃ |
| Температура кипения | 57°C |
| плотность | 1,18 g/cm3 |
| давление пара | 227 at 25 °C (quoted, Howard, 1990) |
| показатель преломления | 1.42213 (20℃) |
| Fp | -6°C |
| температура хранения | Refrigerator |
| растворимость | Miscible with ethanol (U.S. EPA, 1985) |
| Относительная полярность | 0.269 |
| Растворимость в воде | 5.057g/L(25 ºC) |
| Мерк | 3810 |
| БРН | 1696901 |
| констант закона Генри | 4.84 at 25 °C (batch air stripping-GC, Bobadilla et al., 2003) |
| Пределы воздействия | NIOSH REL: TWA 100 ppm (400 mg/m3), IDLH 3,000 ppm; OSHA PEL: TWA 100 ppm; ACGIH TLV: TWA 100 ppm (adopted). |
| Диэлектрическая постоянная | 10.0 |
| Стабильность | Stable. Highly flammable. Vapour/gas mixtures explosive. Incompatible with plastics, many organic materials. Reacts with metals, oxidizing agents. |
| Справочник по базе данных CAS | 75-34-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 10 |
| FDA UNII | 0S989LNA44 |
| Предложение 65 Список | 1,1-Dichloroethane |
| Система регистрации веществ EPA | 1,1-Dichloroethane (75-34-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi,F,Xn,T | |||||||||
| Заявления о рисках | 11-22-36/37-52/53-39/23/24/25-23/24/25 | |||||||||
| Заявления о безопасности | 16-23-26-36-61-45-36/37-7 | |||||||||
| РИДАДР | 2362 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 100 ppm (400 mg/m3) (Chloroethanes) | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KI0175000 | |||||||||
| Примечание об опасности | Flammable/Irritant | |||||||||
| TSCA | T | |||||||||
| Класс опасности | 3.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 2903190090 | |||||||||
| Банк данных об опасных веществах | 75-34-3(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for rats 725 mg/kg (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 3,000 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
1,1-ДИХЛОРЭТАН химические свойства, назначение, производство
Описание
Chlorinated aliphatics as a class are known to cause central nervous system (CNS) depression and respiratory tract and dermal irritation when humans are exposed by inhalation to sufficiently high concentrations. In the past, 1,1-dichloroethane was used as an anesthetic; however, this use was discontinued due to the risk of induction of cardiac arrhythmia in humans. Crystal precipitations and obstruction in the renal tubule lumina and increases in serum urea and creatinine were observed in cats exposed to this compound for weeks. However, these effects were not observed in rats, guinea pigs, or rabbits. However, kidney effectswere observed inmice administered a lethal intraperitoneal injection; the effects included increased glucose and protein in the urine and tubular swelling. The toxicological significance of the nephrotoxicity observed in cats and the mice with regard to human health is not known given the small number of animals tested (cats).The detectionof 1,1-dichoroethane or itsmetabolites in blood and urine cannot predict the type of health effects that might develop from that exposure; because 1,1-dichloroethane and its metabolites leave the body fairly rapidly, the tests need to be conducted within hours to days after exposure.Химические свойства
Also ethylidene chloride,CH3CHCl2 is a colorless,neutral,mobile liquid with an aromatic ethereal odor and saccharin taste. Soluble in alcohol, ether,fixed and volatile oils and very sparingly soluble in water. It is used as an extraction solvent and fumigant.Физические свойства
1,1-Dichloroethane is a clear, colorless, oily liquid with a chloroform-like odor. It is more polar than trans but less polar than cis form.Two chlorine atoms are not on the same side of the plane,hence the net dipole is going to be lesser than cis form.Использование
1,1-Dichloroethane is used as a chemical solvent in the preparation of precursors of quinolizine, isoquinoline and indole alkaloids.Общее описание
A colorless liquid with an ether-like odor. Slightly soluble in water and slightly denser than water. Flash point below 70°F. Vapors denser than air. Used to make other chemicals.Реакции воздуха и воды
Highly flammable. Slightly soluble in water.Профиль реактивности
1,1-DICHLOROETHANE can react vigorously with oxidizing materials. 1,1-DICHLOROETHANE is incompatible with strong bases. Contact with strong caustics will cause formation of flammable and toxic gas. 1,1-DICHLOROETHANE will attack some forms of plastics, rubber and coatings.Опасность
Toxic. Eye and upper respiratory tract irritant; kidney and liver damage. Questionable carcinogen.Угроза здоровью
INHALATION: Irritation of respiratory tract. Salivation, sneezing, coughing, dizziness, nausea, and vomiting. EYES: Irritation, lacrimation, and reddening of conjunctiva. SKIN: Irritation. Prolonged or repeated skin contact can produce a slight burn. INGESTION: Ingestion incidental to industrial handling is not considered to be a problem. Swallowing of substantial amounts could cause nausea, vomiting, faintness, drowsiness, cyanosis, and circulatory failure.Профиль безопасности
Moderately toxic by ingestion. Experimental teratogenic effects. Questionable carcinogen with experimental tumorigenic data. Liver damage reported in experimental animals. A very dangerous fire hazard and moderate explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, water, foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of phosgene and Cl-Возможный контакт
It is used as a solvent; cleaning and degreasing agent; as well as in organic synthesis as an intermediateКанцерогенность
The EPA 2010 classifies 1,1- dichloroethane in group C, a possible human carcinogen, based on no human data and limited evidence of carcinogenicity in two animal species (rats and mice) as shown by an increased incidence of mammary gland adenocarcinomas and hemangiosarcomas in female rats and an increased incidence of hepatocellular carcinomas and benign uterine polyps in mice. The EPA offers no estimate of carcinogenic risk from inhalation or oral exposure. The EPA states (IRIS) that because of similarities in structure and target organs, the carcinogenic evidence for 1,2-dichloroethane is supportive of the classification of 1,1-dichloroethane in group C, a possible human carcinogen. The EPA considers the animal carcinogenicity “limited.”Перевозки
UN2362 1,1-Dichloroethane, Hazard Class: 3; Labels: 3-Flammable liquidМетоды очистки
Shake it with conc H2SO4 or aqueous KMnO4, then wash it with water, saturated aqueous NaHCO3, again with water, dry with K2CO3 and distil it from CaH2 or CaSO4. Store it over silica gel. [Beilstein 1 IV 130.]Несовместимости
Vapor may form explosive mixture with air. Reacts violently with strong oxidizers, alkali metals; earth-alkali metals; powdered metals; causing fire and explosion hazard. Contact with strong caustic will produce flammable and toxic acetaldehyde gas. Attacks aluminum, iron. Attacks some plastics (including polyethylene) and coatings.Утилизация отходов
Incineration; preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal1,1-ДИХЛОРЭТАН запасные части и сырье
сырьё
1of3
запасной предмет
1of2
1,1-ДИХЛОРЭТАН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8619521488211 | China | 2558 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-85511178; +86-85511178; |
China | 35425 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 |