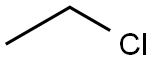
Хлорэтан
- английское имяCHLOROETHANE
- CAS №75-00-3
- CBNumberCB5227785
- ФормулаC2H5Cl
- мольный вес64.51
- EINECS200-830-5
- номер MDLMFCD00000961
- файл Mol75-00-3.mol
| Температура плавления | −139 °C(lit.) |
| Температура кипения | 12.3 °C(lit.) |
| плотность | 0.89 g/mL at 25 °C(lit.) |
| плотность пара | 2.22 (vs air) |
| давление пара | 32.29 psi ( 55 °C) |
| показатель преломления | 1.3676 |
| Fp | <−30 °F |
| температура хранения | 2-8°C |
| растворимость | Soluble in ethanol, ether (U.S. EPA, 1985); miscible with chlorinated hydrocarbons such as chloroform, carbon tetrachloride, and tetrachloroethane. |
| форма | Colorless gas |
| цвет | Colorless to Almost colorless |
| Запах | Ethereal; pungent, ethereal; ether-like. |
| Пределы взрываемости | 3.6-14.8% (v/v) |
| Растворимость в воде | 5.074g/L(20 ºC) |
| Мерк | 14,3782 |
| констант закона Генри | 7.59, 9.58, 11.0, 12.1(x 10-3 atm?m3/mol), and 14.3 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al., 1988) |
| Пределы воздействия | TLV-TWA 1000 ppm (~2600 mg/m3) (ACGIH, MSHA, NIOSH, and OSHA); IDLH 20,000 ppm (NIOSH). |
| Стабильность | Stable. Highly flammable - may form explosive mixtures with air. Incompatible with strong oxidizing agents, alkali metals and their alloys. |
| Справочник по базе данных CAS | 75-00-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5-8 |
| FDA UNII | 46U771ERWK |
| Предложение 65 Список | Chloroethane (Ethyl Chloride) |
| Код УВД | N01BX01 |
| МАИР | 3 (Vol. 52, 71) 1999 |
| Система регистрации веществ EPA | Chloroethane (75-00-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | F+,Xn,T,F | |||||||||
| Заявления о рисках | 45-11-20/21/22-36/37/38-52/53-40-12-39/23/24/25-23/24/25-67-66-22-19-38 | |||||||||
| Заявления о безопасности | 9-16-33-36/37-61-45-7-29-36/37/39-26-53 | |||||||||
| РИДАДР | UN 1993 3/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | KH7525000 | |||||||||
| F | 4.5-31 | |||||||||
| Температура самовоспламенения | 966 °F | |||||||||
| кода HS | 2903.11.0020 | |||||||||
| Класс опасности | 2.1 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 75-00-3(Hazardous Substances Data) | |||||||||
| Токсичность | LC50 (inhalation) for mice 146 gm/m3/2-h, rats 160 gm/m3/2-h (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 3,800 ppm [10% LEL] | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H220:Чрезвычайно легковоспламеняющийся газ.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
Хлорэтан химические свойства, назначение, производство
Химические свойства
colourless gasФизические свойства
Clear, colorless gas or liquid with a pungent or faint, sweetish ether-like odor. When spilled, ethyl chloride evaporates quickly. Odor threshold concentration is 4.2 ppm (quoted, Amoore and Hautala, 1983).Использование
Chloroethane is a useful reactant in organic synthesis.Показания
Chlorethane (ethyl chloride) is a highly flammable liquid that acts as a topical vapocoolant to control pain associated with minor surgical procedures.When applied as a spray, the product produces freezing of superficial tissues to ?20?C, which results in insensitivity of peripheral nerve endings and local anesthesia that is maintained up to 1 minute. Other coolant sprays can be used with the same effect.Методы производства
Ethyl Chloride can be synthesized by treatment of ethyl alcohol with HCl, cleavage of diethylether with HCl in the presence of a catalyst (ZnCl2), chlorination of ethane or hydrochlorination of ethylene. The latter is the choice of industry. The reaction is carried out at 125 °F and 125 psi in the presence of AlCl3, which is dissolved in ethyl chloride.Определение
A highly reactive manmade volatile organic com- pound that is highly reactive in the atmosphere. It readily reacts with oxidizing agents to release the chlorine atoms which, circulate and cause tropo- spheric ozone to decompose.Общее описание
A clear colorless gas with a pungent odor. Flash point -58°F. Boiling point 54°F. Less dense than water and insoluble in water. Vapors are heavier than air. Under prolonged exposure to fire or heat the containers may rupture violently and rocket.Ethyl chloride is used as a solvent for oils,resins,and waxes. It is used in medicine and as an intermediate in synthesis.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
CHLOROETHANE is heat sensitive. CHLOROETHANE will hydrolyze in the presence of alkalis and water. CHLOROETHANE reacts with water or steam to produce toxic and corrosive fumes. CHLOROETHANE can also react vigorously with oxidizing materials. The vapor forms highly flammable mixtures with air. A mixture of CHLOROETHANE with potassium is shock-sensitive. Contact with chemically active metals such as Na, K, Ca, powdered Al, Zn and Mg may result in violent reactions.Опасность
Highly flammable, severe fire and explosion risk; flammable limits in air 3.8–15.4%. Irritant to eyes. Questionable carcinogen.Угроза здоровью
Vapor causes drunkenness, anesthesia, possible lung injury. Liquid may cause frostbite on eyes and skin.Химическая реактивность
Reactivity with Water: No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Профиль безопасности
Suspected carcinogen with experimental carcinogenic and neoplastigenic data. Mildly toxic by inhalation. An irritant to sh, eyes, and mucous membranes. The liquid is harmful to the eyes and can cause some irritation. In the case of guinea pigs, the symptoms attending exposure are similar to those caused by methyl chloride, except that the signs of lung irritation are not as pronounced. It gives some warning of its presence because it is irritating, but it is possible to tolerate exposure to it until one becomes unconscious. It is the least toxic of all the chlorinated hydrocarbons. It can cause narcosis, although the effects are usually transient. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Severe explosion hazard when exposed to flame. Reacts with water or steam to produce toxic and corrosive fumes. Incompatible with potassium. To fight fire, use carbon dioxide. When heated to decomposition it emits toxic fumes of phosgene and Cl-. See also CHLORINATED HYDROCARBONS, ALIPHATIC.Возможный контакт
Ethyl chloride is used as an ethylating agent in the manufacture of tetraethyl lead, dyes, drugs, and ethyl cellulose; as a pharmaceutical, solvent; alkylating agent; as a refrigerant and as a local anesthetic (freezing).Канцерогенность
The EPA has not made a carcinogenicity assessment as yet. However, the State of California reviewed the carcinogenicity information.CalEPA, using the NTP study, developed a cancer potency estimate of 4.7E-3 per mg/kg/day and defined a No Significance Risk Level (NSRL) of 1 50 μg/day.
Increased cancer of the uterus of female mice has been produced by exposure to 15,000 ppm, but lower concentrations have not been studied. Rats and mice were exposed to 0 or 15,000 ppm of ethyl chloride in an NTP 2-year study with mixed results. Results in male rats were considered equivocal based on a combined total of five skin tumors versus none in the control male rats. Likewise, female rats’ results were considered equivocal because three astrocytomas were found versus none in the female control rats. The male mouse group had such poor survival that it was deemed an inadequate study although combined alveolar/bronchiolar adenomas and carcinomas were reported (10/48 versus 5/50 in the control male rats). Female mice exposed to 15,000 ppm had clear evidence of an effect, for 43/50 mice had endometrial uterine carcinomas versus 0/49 in the female control mice. In addition, there was a suggestion of an increase in combined hepatocellular adenomas and carcinomas in the female mice (8/48 exposed versus 3/49 control). There is clear evidence for carcinogenicity in female B6C3F1 mice and equivocal evidence in male and female F344/N rats (high incidence of uterine carcinomas.)
Экологическая судьба
Photolytic. The rate constant for the reaction of chloroethane and OH radicals in the atmosphere at 300 K is 2.3 x 10-11 cm3/molecule?sec (Hendry and Kenley, 1979). At 296 K, a photooxidation rate constant of 3.9 x 10-13 cm3/molecule?sec was reported (Howard and Evenson, 1976). The estimated tropospheric lifetime is 14.6 d (Nimitz and Skaggs, 1992).Chemical/Physical. Under laboratory conditions, chloroethane hydrolyzed to ethanol (Smith and Dragun, 1984). An estimated hydrolysis half-life in water at 25 °C and pH 7 is 38 d, with ethanol and HCl being the expected end-products (Mabey and Mill, 1978). Based on a measured hydrolysis rate constant of 5.1 x 10-7 at 25 °C and pH 7, the half-life is 2.6 yr (Jeffers and Wolfe, 1996).
In air, formyl chloride is the initial photooxidation product (U.S. EPA, 1985). In the presence of water, formyl chloride hydrolyzes to HCl and carbon monoxide (Morrison and Boyd, 1971).
Burns with a smoky, greenish flame releasing hydrogen chloride (Windholz et al., 1983).
In the laboratory, the evaporation half-life of chloroethane (1 mg/L) from water at 25 °C using a shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 23.1 min (Dilling, 1977).
At influent concentrations of 1.0, 0.1, and 0.01 mg/L, the GAC adsorption capacities at pH 5.3 were 0.59, 0.07, and 0.007 mg/g, respectively (Dobbs and Cohen, 1980).
Перевозки
UN1037 Ethyl chloride, Hazard Class: 2.1; Labels: 2.1-Flammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Методы очистки
Pass ethyl chloride through absorption towers containing, successively, conc H2SO4, NaOH pellets, P2O5 on glass wool, or soda-lime, CaCl2, P2O5. Condensed it into a flask containing CaH2 and fractionally distil it. It has also been purified by illumination in the presence of bromine at 0o using a 1000W lamp, followed by washing, drying and distilling. [Beilstein 1 IV 124.]Несовместимости
Flammable gas. Slow reaction with water; forms hydrogen chloride gas. Contact with moisture (water, steam) forms hydrochloric acid and/or fumes of hydrogen chloride. May accumulate static electrical charges, and may cause ignition of its vapors. May form explosive mixture with air. Contact with chemically active metals: aluminum, lithium, magnesium, sodium, potassium, zinc may cause fire and explosions. Attacks some plastics and rubber.Утилизация отходов
Return refillable compressed gas cylinders to supplier. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.Хлорэтан запасные части и сырье
сырьё
1of3
запасной предмет
- Flufenoxuron
- polysulfone anion exchange membrane
- Ламивудин
- Ломефлоксацин
- 4-Choro-2(3H)-benzothiazolone
- 3-диэтиламинофенол
- Diethyl 2,3-dichlorobutanedioate
- 2-этоксибензамид
- 1-МЕТИЛ-4-ЭТОКСИКАРБОНИЛ-ПИРАЗОЛ-5-СУЛЬФОНИЛХЛОРИД
- Флуразепам
1of7
Хлорэтан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| 0086 18039857276 18039857276 |
CHINA | 2783 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| United States | 2344 | 58 | ||
| 13000000000 | United States | 1634 | 58 |
Хлорэтан Обзор)
- 2-хлорэтил хлорформиа
- Хлорид 3-бромбензоил
- O-фталоил дихлорид
- 2,4-дифторбензоил хлорид
- Хлорид Pentafluorobenzoyl
- 2-фторбензоил хлорид
- O-толуил хлорид
- 3-хлорбензоил хлорид
- 2,6-дифторбензоил хлорид
- 2-бромбензоил хлорид
1of4