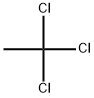
1,1,1-TРИХЛОЭТАН
- английское имя1,1,1-Trichloroethane
- CAS №71-55-6
- CBNumberCB3701849
- ФормулаC2H3Cl3
- мольный вес133.4
- EINECS200-756-3
- номер MDLMFCD00000806
- файл Mol71-55-6.mol
химическое свойство
| Температура плавления | −35 °C(lit.) |
| Температура кипения | 74-76 °C(lit.) |
| плотность | 1.336 g/mL at 20 °C(lit.) |
| плотность пара | 4.6 (vs air) |
| давление пара | 100 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 11 °C |
| температура хранения | 0-6°C |
| растворимость | Sparingly soluble in ethyl alcohol; freely soluble in carbon disulfide, benzene, ethyl ether, methanol, carbon tetrachloride (U.S. EPA, 1985), and many other organic solvents. |
| форма | Fluid |
| Растворимость в воде | 1.4 g/L (20 ºc) |
| Мерк | 13,9710 |
| констант закона Генри | 2.77 at 40 °C, 4.27 at 50 °C, 6.31 at 60 °C, 7.91 at 70 °C, 8.98 at 80 °C (headspace-GC, Vane et al., 2001) |
| Пределы воздействия | TLV-TWA 350 ppm (~1900 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 450 ppm (~2450 mg/m3) (ACGIH); IDLH 1000 ppm (NIOSH). |
| Диэлектрическая постоянная | 7.9(19℃) |
| ИнЧИКей | UOCLXMDMGBRAIB-UHFFFAOYSA-N |
| Стандарт первичной питьевой воды EPA | MCL:0.2,MCLG:0.2 |
| LogP | 2.490 |
| Справочник по базе данных CAS | 71-55-6(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | 1,1,1-TRICHLOROETHANE |
| FDA 21 CFR | 175.105; 177.1650 |
| Рейтинг продуктов питания EWG | 4-5 |
| FDA UNII | 113C650IR1 |
| МАИР | 3 (Vol. 20, Sup 7, 71) 1999 |
| Справочник по химии NIST | Ethane, 1,1,1-trichloro-(71-55-6) |
| Система регистрации веществ EPA | 1,1,1-Trichloroethane (71-55-6) |
больше
| Коды опасности | Xn,N,T,F | |||||||||
| Заявления о рисках | 20-59-66-40-19-39/23/24/25-23/24/25-11-36/38 | |||||||||
| Заявления о безопасности | 24/25-59-61-9-46-16-45-36/37-7-26 | |||||||||
| РИДАДР | UN 2831 6.1/PG 3 | |||||||||
| OEL | Ceiling: 350 ppm (1900 mg/m3) [15-minute] (Chloroethanes) | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KJ2975000 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29031910 | |||||||||
| Банк данных об опасных веществах | 71-55-6(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for dogs 750 mg/kg, guinea pigs 9,470 mg/kg, mice 11,240 mg/kg, rats 10,300 mg/kg, rabbits 5,660 mg/kg (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 700 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H420:Разрушает озоновый слой.
-
оператор предупредительных мер
P502:Обратиться к изготовителю или поставщику для получения информации о вторичной переработке или утилизации.
1,1,1-TРИХЛОЭТАН химические свойства, назначение, производство
Описание
1,1,1-Trichloroethane (1,1,1-TCE) was first identified in 1840 by Henri Victor Regnault, a French chemist and physicist. 1,1,1- TCE is a synthetic chemical that is released to the environment primarily by human industrial activity such as by-process and fugitive emissions during its manufacture, formulation, and use in both consumer and industrial products, which can then undergo thermal and photochemical chlorination. 1,1,1-TCE was originally introduced as a replacement for other chlorinated and flammable solvents like carbon tetrachloride. Although trichloroethane was formerly used extensively in a range of industrial applications and consumer products, including such products as adhesives and adhesive cleaners, lubricants, general purpose liquid cleaners and spray degreasers, oven cleaners, spot removers, shoe polish, and fabric finishes, and as a precursor for hydrofluorocarbons, it is no longer used in common household products. 1,1,1-TCE was one of the compounds addressed by the Montreal Protocol in 1987, which stipulates that the production and consumption of these potentially ozone-depleting substances in the stratosphere were to be phased out. Under this agreement, the final phase out for developed countries for 1,1,1-TCE was 1996, with selected exceptions for existing stocks and essential uses; developing countries have until 2015 for their ban to take effect.Химические свойства
1,1,1-Trichloroethane is a colorless liquid. It has an odor similar to chloroform. The Odor Threshold is 120 ppm (NJ) or 400 ppm (NY).Физические свойства
Colorless, watery liquid with a dusty, sooty or polish-type odor similar to chloroform. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor was detected were 20,000 and 2,200 μg/L, respectively. At 25 °C, the lowest concentration at which a taste was detected was 1,500 μg/L, respectively (Young et al., 1996). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.47 and 0.32 mg/L, respectively (Alexander et al., 1982).Использование
1,1,1-Trichloroethane is used as a cleaningsolvent for cleaning metals and plastic molds.Методы производства
Most commercial methyl chloroform, which is sold under several trade names, contains inhibitors to prevent reaction of the solvent with aluminum and alloys. This reaction produces hydrogen chloride and in confined vessels may produce high pressures.Определение
ChEBI: A member of the class of chloroethanes carrying three chloro substituents at position 1.Общее описание
A colorless liquid with a sweet, pleasant odor. May irritate skin, eyes and mucous membranes. In high concentrations the vapors may have a narcotic effect. Nonflammable, but may decompose and emit toxic chloride fumes if exposed to high temperatures. Used as a solvent.Реакции воздуха и воды
Insoluble in water. Absorbs some water.Профиль реактивности
1,1,1-Trichloroethane decomposes in the presence of chemically active metals. This includes aluminum, magnesium and their alloys. 1,1,1-Trichloroethane will react violently with dinitrogen tetraoxide, oxygen, liquid oxygen, sodium and sodium-potassium alloys. 1,1,1-Trichloroethane will also react violently with acetone, zinc and nitrates. 1,1,1-Trichloroethane can react with sodium hydroxide. 1,1,1-Trichloroethane is incompatible with strong oxidizers and strong bases. Mixtures with potassium or its alloys are shock-sensitive and may explode on light impact. 1,1,1-Trichloroethane can react with an aqueous suspension of calcium hydroxide, and with chlorine in sunlight. 1,1,1-Trichloroethane will attack some forms of plastics, rubber and coatings. Upon contact with hot metal or on exposure to ultraviolet radiation, 1,1,1-Trichloroethane will decompose to form irritant gases. A cobalt/molybdenum-alumina catalyst will generate a substantial exotherm on contact with its vapor at ambient temperatures. Hazardous reactions also occur with (aluminum oxide + heavy metals). .Угроза здоровью
The oral and inhalation toxicity of 1,1,1-trichloroethane is of low order in animalsand humans. It is an anesthetic at highconcentrations. Exposure to its vapors at a1.5% concentration in air may be lethal tohumans. Death may result from anesthesiaand/or cardiac sensitization. Prolonged skincontact may cause defatting and reddeningof eyes. Vapors are irritant to the eyes andmucous membranes.The acute oral toxicity is low in testanimals. The oral LD50 values in rabbitsand guinea pigs are 5660 and 9470 mg/kg,respectively (NIOSH 1986). The carcino genicity of this compound in animals andhumans is not known.
Пожароопасность
Special Hazards of Combustion Products: Toxic and irritating gases are generated in fires.Контактные аллергены
Trichloroethane is a solvent that has wide applications in industry, such as for cold type metal cleaning and in cleaning plastic molds. It is mainly an irritant, but can also provoke allergic contact dermatitis.Профиль безопасности
Poison by intravenous route. Moderately toxic by ingestion, inhalation, skin contact, subcutaneous, and intraperitoneal routes. An experimental teratogen. Human systemic effects by ingestion and inhalation: conjunctiva irritation, hallucinations or distorted perceptions, motor activity changes, irritability, aggression, hypermotility, diarrhea, nausea or vomiting and other gastrointestinal changes. Experimental reproductive effects. Questionable carcinogen. Mutation data reported. A human skin irritant. An experimental skin and severe eye irritant. Narcotic in high concentrations. Causes a proarrhythmic activity that sensitizes the heart to epinephrine-induced arrhythmias. This sometimes will cause cardlac arrest, particularly when this material is massively inhaled as in drug abuse for euphoria.Under the proper conditions it can undergo hazardous reactions with aluminum oxide + heavy metals, dinitrogen tetraoxide, inhbitors, metals (e.g., magnesium, aluminum, potassium, potassium-sodium alloy), sodium hydroxide, N2O4, oxygen. When heated to decomposition it emits toxic fumes of Cl-. Used as a cleaning solvent, as a chemical intermediate to produce vinylidene chloride, and as a propellant in aerosol cans.
Возможный контакт
1,1,1-Trichloroethane is used as a cleaning solvent, chemical intermediate for vinylidene chloride. In liquid form it is used as a degreaser and for cold cleaning, dip-cleaning; and bucket cleaning of metals. Other industrial applications of 1,1,1-trichlroethane’s solvent properties include its use as a dry-cleaning agent; a vapor degreasing agent; and a propellant. In recent years, 1,1,1-trichloroethane has found wide use as a substitute for carbon tetrachloride.Канцерогенность
IRIS provides a cancer descriptor of “inadequate information to assess carcinogenic potential.” This is based on inconclusive epidemiologic studies. A 2 year inhalation bioassay showed no treatment- related increase in tumors in rats and mice. The two available oral cancer bioassays in rats and mice are inadequate for evaluation of cancer potential. The compound has been shown to be rather negative in short-term tests for genotoxicity.NCI tested rats and mice by oral and inhalation routes, but the results were questionable. Quast et al. exposed 96 Sprague–Dawley rats of both sexes to 875 or 1750 ppm 1,1,1-trichloroethane vapor for 6 h/ day, 5 days/week for 12 months, followed by an additional 19 month observation period. The only significant sign of toxicity was an increased incidence of focal hepatocellular alterations in female rats at the highest dosage. Neither was it evident that a maximum tolerated dose was used nor was a range-finding study conducted. No significant dose-related neoplasms were reported, but these dose levels were below those used in the NCI study.
In another study, Quast et al. used an inhibited formulation of 1,1,1-trichloroethane. Fischer 344 rats and B6C3F1 mice of both sexes were exposed to 0, 150, 500, or 1500 ppm 6 h/day, 5 days/week for 2 years. The authors indicate that there were no indications of an oncogenic effect on rats or mice following 2 years of exposure to the 1,1,1- trichloroethane formulation and a NOAEL of 500 ppm for adverse effect of any kind. The ATSDR reviewed this information (52) and determined that the study adequately demonstrated negative evidence of carcinogenicity in animals by lifetime inhalation up to 1500 ppm.
Перевозки
UN2831 1,1,1-Trichloroethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Методы очистки
Wash it successively with conc HCl (or conc H2SO4), aqueous 10% K2CO3 (Na2CO3), aqueous 10% NaCl, dry it with CaCl2 or Na2SO4, and fractionally distil it. It can contain up to 3% dioxane as preservative. This is removed by washing successively with 10% aqueous HCl, 10% aqueous NaHCO3 and 10% aqueous NaCl, and distilling over CaCl2 before use. [Beilstein 1 IV 138.]Несовместимости
Not flammable under normal conditions. However, in close or closed spaces, it may form a dangerously explosive atmosphere. See also fireextinguishing section. Strong caustics; strong oxidizers; chemically active metals, such as aluminum, magnesium powder; sodium, potassium. Reacts slowly with water forming hydrochloric acid. Upon contact with hot metal or exposure to UV radiation, it will decompose to form hydrochloric acid, phosgene and dichloroacetylene. Forms shocksensitive mixtures with potassium or its alloys. Attacks natural rubber.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. As an alternative to disposal, trichloroethane may be recovered from waste gases and liquids from various processes and recycled.