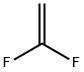
1,1-дифторэтен
- английское имя1,1-DIFLUOROETHYLENE
- CAS №75-38-7
- CBNumberCB7240544
- ФормулаC2H2F2
- мольный вес64.03
- EINECS200-867-7
- номер MDLMFCD00000448
- файл Mol75-38-7.mol
| Температура плавления | −144 °C(lit.) |
| Температура кипения | −83 °C(lit.) |
| плотность | 0,617 g/cm3 |
| плотность пара | 2.2 (vs air) |
| давление пара | 518 psi ( 21 °C) |
| показатель преломления | 1.3225 (estimate) |
| Fp | <-60°C |
| Пределы взрываемости | 21.3% |
| БРН | 1733321 |
| Диэлектрическая постоянная | 3.0(Ambient) |
| Справочник по базе данных CAS | 75-38-7(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | 1,1-DIFLUOROETHENE |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 3C1IX2905B |
| МАИР | 3 (Vol. 39, Sup 7, 71) 1999 |
| Система регистрации веществ EPA | Vinylidene fluoride (75-38-7) |
| Коды опасности | F+,F | |||||||||
| Заявления о рисках | 12 | |||||||||
| Заявления о безопасности | 16-7/9 | |||||||||
| РИДАДР | UN 1959 2.1 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 1 ppm, Ceiling: 5 ppm [use 1910.1017] | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KW0560000 | |||||||||
| F | 4.5 | |||||||||
| Температура самовоспламенения | 1184 °F | |||||||||
| Примечание об опасности | Flammable | |||||||||
| TSCA | T | |||||||||
| Класс опасности | 2.1 | |||||||||
| кода HS | 2903590090 | |||||||||
| Банк данных об опасных веществах | 75-38-7(Hazardous Substances Data) | |||||||||
| Токсичность | LCLo inhalation in guinea pig: 800ppb/4H | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H220:Чрезвычайно легковоспламеняющийся газ.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P377:При воспламенении газа в случае утечки не тушить, если это сопряжено с риском.
P381:Устранить источники воспламенения, если это не сопряжено с риском.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
1,1-дифторэтен химические свойства, назначение, производство
Химические свойства
Colorless gas; faint ethereal odor. Slightly soluble in water; soluble in alcohol and ether.Использование
1,1-Difluoroethylene is used in the manufacture of polyvinylidene fluoride for its use as a thermal, chemical, and ultraviolet light–resistant agent, and as an anticorrosive agent. The monofilament form is used as filter cloth in the pulp and paper industry. Due to its high melting temperature, it can be used as an insulator. Some of its copolymers are used for their heat- and moisture-resistant properties, primarily in industrial, aerospace, and automotive applications.Общее описание
1,1-DIFLUOROETHYLENE (or vinylidene fluoride) is a colorless gas which is flammable in the ranges of 5.5 to 21%. 1,1-DIFLUOROETHYLENE is toxic by inhalation and contact. 1,1-DIFLUOROETHYLENE is slightly soluble in water and soluble in alcohol and ether. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.Профиль реактивности
1,1-DIFLUOROETHYLENE is sensitive to heat. 1,1-DIFLUOROETHYLENE is incompatible with oxidizers. 1,1-DIFLUOROETHYLENE can react violently with hydrogen chloride. Alkyl boron and alkyl hyponitrite compounds initiate polymerization. 1,1-DIFLUOROETHYLENE will form peroxides on exposure to pure oxygen. .Угроза здоровью
Vapors may cause dizziness or asphyxiation without warning. Some may be toxic if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases.Пожароопасность
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Silane will ignite spontaneously in air. May polymerize explosively when heated or involved in a fire. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.Профиль безопасности
Suspected carcinogen with experimental neoplastigenic data. Mildly toxic by inhalation. Mutation data reported. A very dangerous fire hazard when exposed to heat, flame, or oxidzers. Explosive in the form of vapor when exposed to heat or flame. Violent reaction with hydrogen chloride when heated under pressure. To fight fire, stop flow of gas. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES.Возможный контакт
Vinylidene fluoride is used in the formulation of many polymers and copolymers, such as chlorotrifluoroethylene-vinylidene fluoride (Kel F), perfluoropropylene-vinylidene fluoride (Viton, Fluorel); polyvinylidene fluoride; and hexafluoropropylene-tetra-fluoroethylene- vinylidene fluoride; elastomeric copolymers. It is also used as a chemical intermediate in organic synthesis. NIOSH has estimated 32,000 workers are exposed annually.Экологическая судьба
Oxidative metabolism of 1,1-Difluoroethylene is mediated by the hepatic microsomal monooxygenase to form epoxide and the inactivation of hemeprotein.Перевозки
UN1959 Vinylidene fluoride shipped as 1,1-Difluoroethylene or Refrigerant gas R-1132a, Hazard Class: 2.1; Labels: 2.1-Flammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Несовместимости
idizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, aluminum chloride. Capable of forming unstable peroxides, which can cause explosive polymerization. It can re act violently with hydrogen chloride. Alkyl boron and alkyl hyponitrite compounds initiate polymerization. It will form peroxides on exposure to pure oxygen. May accumulate static electricity, and cause ignition of its vapors.Утилизация отходов
Return refillable compressed gas cylinders to supplier.1,1-дифторэтен запасные части и сырье
1,1-дифторэтен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| 18017061086 | CHINA | 5600 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +8618950047208 | China | 43416 | 58 |