FLUOROSULFONIC ACID
- русский язык имя
- английское имяFLUOROSULFONIC ACID
- CAS №7789-21-1
- CBNumberCB7728488
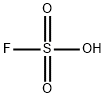
- ФормулаFHO3S
- мольный вес100.07
- EINECS232-149-4
- номер MDLMFCD00066174
- файл Mol7789-21-1.mol
| Температура плавления | −87.3 °C(lit.) |
| Температура кипения | 165.5 °C(lit.) |
| плотность | 1.726 g/mL at 25 °C(lit.) |
| плотность пара | 3.5 (vs air) |
| давление пара | 2.5 mm Hg ( 25 °C) |
| температура хранения | 2-8°C |
| растворимость | reacts with H2O |
| пка | -6.29±0.15(Predicted) |
| форма | colorless liquid |
| цвет | colorless |
| Растворимость в воде | hydrolyzes violently in H2O; reddish?brown color in acetone [MER06] |
| Мерк | 13,4206 |
| Стабильность | Stable. May corrode glass. Incompatible with many metals, bases. |
| Справочник по базе данных CAS | 7789-21-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | PPX0648643 |
| Система регистрации веществ EPA | Fluorosulfuric acid (7789-21-1) |
| UNSPSC Code | 12352300 |
| NACRES | NA.23 |
| Коды опасности | C,T |
| Заявления о рисках | 20-35 |
| Заявления о безопасности | 26-45 |
| РИДАДР | UN 1777 8/PG 1 |
| WGK Германия | 1 |
| RTECS | LP0715000 |
| Примечание об опасности | Corrosive/Toxic |
| Класс опасности | 8 |
| Группа упаковки | I |
| Банк данных об опасных веществах | 7789-21-1(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
FLUOROSULFONIC ACID химические свойства, назначение, производство
Описание
Fluorosulphuric acid (FSO3H) is a free-flowing colourless fuming liquid. It is soluble in polar organic solvents such as acetic acid, ethyl acetate, and nitrobenzene but poorly soluble in nonpolar solvents such as alkanes. FSO3H is corrosive to metals and body tissues, corrodes glass, and is incompatible with many metals and bases. With its strong acidity, FSO3H dissolves almost all organic compounds that are even weak proton acceptors. FSO3H hydrolyses slowly to HF and sulphuric acid (H2SO4). The related triflic acid (CF3SO3H) retains the high acidity of FSO3H but is more hydrolytically stable. FSO3H is one of the strongest known simple Bronsted acids. FSO3H is useful for regenerating mixtures of HF and H2SO4 for etching lead glass. FSO3H isomerises alkanes and the alkylation of hydrocarbons with alkenes, although it is unclear if such applications are of commercial importance. FSO3H is used as a catalyst in organic synthesis and in electroplating and as a fluorinating agent and as a laboratory fluorinating agent. FSO3H has been reported as a highly toxic and corrosive chemical substance. FSO3H is non-combustible but enhances combustion of other substances. It gives off irritating or toxic fumes (or gases) in a fire. FSO3H hydrolyses to release HF. Addition of water to FSO3H causes very violent reactions similar to the addition of water to H2SO4. Addition of FSO3H to water is a much more violent process than addition of H2SO4. The combination of FSO3H and others is categorised as ‘magic acids and super acids’. Very short contact and the fumes of FSO3H cause severe painful burns.Химические свойства
colourless liquidИспользование
Catalyst in organic synthesis, electropolishing, fluorinating agent.Общее описание
A fuming liquid. Boiling point 163°C. Density 1.73 g / cm3. Corrosive to metals and to tissue. Both very short contact and the fumes can cause severe painful burns. Used as a catalyst in organic synthesis, in electroplating and as a fluorinating agent.Реакции воздуха и воды
Fumes in air. Reacts violently with water to form hydrogen fluoride and sulfuric acid mist.Профиль реактивности
FLUOROSULFONIC ACID is very strongly acidic [Merck]. Reacts exothermically with chemical bases (examples: amines, amides, and inorganic hydroxides). These reactions can generate dangerously large amounts of heat in small spaces. Reacts violently with water to generate hydrofluoric acid and sulfuric acid. Reacts with active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. Generates flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. May catalyze (increase the rate of) chemical reactions.Опасность
Extremely irritating to eyes and tissue.Угроза здоровью
Inhalation of fumes causes severe irritation of nose and throat. Contact of liquid with eyes or skin causes very severe burns of mouth and stomach.Профиль безопасности
Probably a poison by inhalation. A corrosive irritant to the skin, eyes, and mucous membranes. See also FLUORIDES, SULFURIC ACID, and FLUOROSULFONATES.FLUOROSULFONIC ACID запасные части и сырье
FLUOROSULFONIC ACID поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0532-86867058 +86-18562606086 |
China | 478 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +8615056975894 | CHINA | 9911 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-85511178; +86-85511178; |
China | 35425 | 58 |