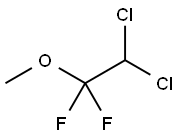
Метоксифлуран
- английское имяmethoxyflurane
- CAS №76-38-0
- CBNumberCB6314297
- ФормулаC3H4Cl2F2O
- мольный вес164.97
- EINECS200-956-0
- номер MDLMFCD00040144
- файл Mol76-38-0.mol
| Температура плавления | -36°C |
| Температура кипения | 103 °C |
| плотность | 1.4262 |
| показатель преломления | 1.386 |
| Fp | 37°C |
| температура хранения | -70°C |
| растворимость | Chloroform (Sparingly) |
| форма | liquid |
| цвет | Clear |
| Биологические источники | rabbit |
| Растворимость в воде | Miscible with alcohol, acetone, chloroform, ether, fixed oils and benzene. Immiscible with water. |
| Мерк | 14,5994 |
| БРН | 1737766 |
| Пределы воздействия | No exposure limit is set. Based on comparison with related compounds, a TLV-TWA of 675 mg/m3 (100 ppm) is recommended. |
| Стабильность | Stability |
| Справочник по базе данных CAS | 76-38-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 30905R8O7B |
| Словарь наркотиков NCI | methoxyflurane |
| Код УВД | N02BG09 |
| Система регистрации веществ EPA | Methoxyflurane (76-38-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.41 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 10 | |||||||||
| Заявления о безопасности | 23-24/25 | |||||||||
| РИДАДР | 3271 | |||||||||
| OEL | Ceiling: 2 ppm (13.5 mg/m3) [60-minute] [*Note: REL for exposure to waste anesthetic gas.] | |||||||||
| RTECS | KN7820000 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 2909191800 | |||||||||
| Банк данных об опасных веществах | 76-38-0(Hazardous Substances Data) | |||||||||
| Токсичность | One of the most potent of the inhalational anesthetics, having a very high blood-gas partition coefficient and low vapor pressure at room temperature. Methoxyflurane is metabolized to a great extent (about 50-70%) in the liver and, as a consequence, there may be release of high concentrations of fluoride, sufficient to exceed the threshold for renal damage. Its use for sustained anesthesia is limited because of this renal toxicity and was discontinued around 1980. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P241:Использовать взрывобезопасное оборудование и освещение.
P308+P311:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Метоксифлуран химические свойства, назначение, производство
Химические свойства
colourless liquidИспользование
Methoxyflurane is a very potent and highly lipid soluble anesthetic agent. Methoxyflurane causes deep sedation and it has been used as a patient controlled analgesic for painful procedures in children. Methoxyflurane is a significant respiratory depressant.Определение
ChEBI: An ether in which the two groups attached to the central oxygen atom are methyl and 2,2-dichloro-1,1-difluoroethyl.Биологические функции
Methoxyflurane (Penthrane) is the most potent inhalational agent available, but its high solubility in tissues limits its use as an induction anesthetic. Its pharmacological properties are similar to those of halothane with some notable exceptions. For example, since methoxyflurane does not depress cardiovascular reflexes, its direct myocardial depressant effect is partially offset by reflex tachycardia, so arterial blood pressure is better maintained. Also, the oxidative metabolism of methoxyflurane results in the production of oxalic acid and fluoride concentrations that approach the threshold of causing renal tubular dysfunction. Concern for nephrotoxicity has greatly restricted the use of methoxyflurane.Общее описание
Methoxyflurane is a volatile liquid (bp=105°C) with a highblood:gas partition coefficient and thus a slow induction andprolonged recovery. Approximately 75% of the drug undergoesmetabolism yielding dichloroacetate, difluoromethoxyacetate,oxalate, and fluoride ions. The intrarenal inorganicfluoride concentration, as a result of renal defluorination, maybe responsible for the nephrotoxicity seen with methoxyflurane.Both the concentration of F- generated and the durationfor which it remained elevated were factors in the developmentof methoxyflurane nephrotoxicity. Methoxyfluranewas removed from the U.S. market in 2000 because of saferalternatives. Both isoflurane and enflurane produce less fluorideion upon metabolism than methoxyflurane.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
2,2-DICHLORO-1,1-DIFLUOROETHYL METHYL ETHER may be sensitive to prolonged exposure to light.Угроза здоровью
Methoxyflurane exhibited low to very lowacute toxicity via inhalation, slightly lowerthan that of ethrane. Oral toxicity was low tomoderate depending on the species. Inhala tion of its vapors at 1.5–2% by volumeconcentrations in air can cause anesthesia inhumans. The toxic symptoms are similar tothose of ethrane, and the target organs areprimarily the central nervous system, kidney,and liver. At subanesthetic concentrations of0.3–0.5% by volume in air, its exposure tohumans for 1 hour resulted in the onset oflow toxicity. The sites of biological effectswere in the kidney.LC50 value, inhalation (mice): 17,500 ppm/2 hr
LD50 value, oral (mammals): 3600 mg/kg
The liquid may be an irritant to theeyes. The teratomeric properties of this com pound were observed in rats and mice. Thesymptoms were embryo deaths and develop mental abnormalities in the urogenital andmusculoskeletal systems.
No carcinogenic actions in animals orhumans have been reported. The histidinereversion–Ames test for mutagenicity wasinconclusive.
Пожароопасность
2,2-DICHLORO-1,1-DIFLUOROETHYL METHYL ETHER is combustible.Клиническое использование
Methoxyflurane is seldom used because of its propensity to cause renal toxicity. It is the most potent agent, and it has the highest solubility in blood. Induction and recovery would be expected to be slow. Chemically, it is rather unstable, and as much as 50% of an administered dose can be metabolized. Toxic metabolites significantly limit its utility as a general anesthetic.Метоксифлуран запасные части и сырье
Метоксифлуран поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +8615986615575 | China | 20342 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +86-16631818819 +86-17736933208 |
China | 9300 | 58 | |
| +8613817748580 | China | 40066 | 58 |