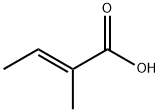
Транс-2,3-диметилакриловая кислота
- английское имяTiglic acid
- CAS №80-59-1
- CBNumberCB4199986
- ФормулаC5H8O2
- мольный вес100.12
- EINECS201-295-0
- номер MDLMFCD00066864
- файл Mol80-59-1.mol
| Температура плавления | 61-64 °C(lit.) |
| Температура кипения | 95-96 °C12 mm Hg(lit.) |
| плотность | 0.969 g/mL at 25 °C(lit.) |
| показатель преломления | nD81 1.4342 |
| FEMA | 3599 | 2-METHYL-TRANS-2-BUTENOIC ACID |
| Fp | 101°C |
| температура хранения | 2-8°C |
| растворимость | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| пка | pK (25°) 5.02 |
| форма | Crystalline Powder and Chunks |
| цвет | White to beige |
| Удельный вес | 0.969 |
| Запах | at 10.00 % in dipropylene glycol. sweet dry spicy woody caramel |
| Odor Type | spicy |
| Биологические источники | synthetic |
| Растворимость в воде | SOLUBLE IN HOT WATER |
| Номер JECFA | 1205 |
| Мерк | 14,9433 |
| БРН | 1236500 |
| ИнЧИКей | UIERETOOQGIECD-ONEGZZNKSA-N |
| LogP | 1.35 |
| Справочник по базе данных CAS | 80-59-1(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | TRANS-2-METHYL-2-BUTENOIC ACID |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | I5792N03HC |
| Справочник по химии NIST | 2-Butenoic acid, 2-methyl-, (E)-(80-59-1) |
| Система регистрации веществ EPA | 2-Butenoic acid, 2-methyl-, (2E)- (80-59-1) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Коды опасности | C,Xi | |||||||||
| Заявления о рисках | 34-36/37/38 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-24/25-27 | |||||||||
| РИДАДР | UN 3261 8/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | GQ5430000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29161980 | |||||||||
| Банк данных об опасных веществах | 80-59-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Транс-2,3-диметилакриловая кислота химические свойства, назначение, производство
Описание
frarc.s-2-Methyl-2-butenoic acid has a sweet, warm, spicy odor. May be synthesized from 2-hydroxy-2-methylbutyronitrile.Химические свойства
WHITE TO BEIGE CRYSTALLINE POWDER AND CHUNKSВхождение
Reported found in celery leaves and stalk, cocoa, mango, dried bonito and Roman chamomile oilИспользование
Tiglic acid is the stable isomer of angelic acid. Tiglic acid was found as glyceride in croton oil, as butyl ester in the oil of the Roman camomile, and as geranyl tiglate in oil of geranium. Tiglic a cid is formed during the charcoaling of maple wood.Определение
ChEBI: A 2-methylbut-2-enoic acid having its double bond in trans-configuration.Общее описание
A white lustrous flaked solid with a fruity or spicy odor. About the same density as water and slightly soluble in water. May burn if heated to high temperatures. May severely irritate skin, eyes, lungs or mucous membranes.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
TIGLIC ACID is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Tiglic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Профиль безопасности
Low toxicity by ingestion and skin contact. A severe skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.Методы очистки
Crystallise it from water. It is steam volatile and is soluble in organic solvents. [Beilstein 2 IV 1552.]Транс-2,3-диметилакриловая кислота запасные части и сырье
Транс-2,3-диметилакриловая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 571-88938639 +8617705817739 |
China | 52849 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 18017610038 | CHINA | 3619 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86-28-82633860; +8618080483897 |
China | 3772 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |