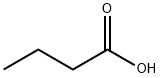
Масляная кислота
- английское имяButyric Acid
- CAS №107-92-6
- CBNumberCB3459186
- ФормулаC4H8O2
- мольный вес88.11
- EINECS203-532-3
- номер MDLMFCD00002814
- файл Mol107-92-6.mol
| Температура плавления | ?6-?3 °C (lit.) |
| Температура кипения | 162 °C (lit.) |
| плотность | 0.964 g/mL at 25 °C (lit.) |
| плотность пара | 3.04 (vs air) |
| давление пара | 0.43 mm Hg ( 20 °C) |
| показатель преломления | n |
| FEMA | 2221 | BUTYRIC ACID |
| Fp | 170 °F |
| температура хранения | Store below +30°C. |
| растворимость | Chloroform (Soluble), Isopropanol (Sparingly), Methanol (Slightly);Miscible with water, Propylene glycol, Glycerin, alcohol and oils. |
| пка | 4.83(at 25℃) |
| форма | Liquid |
| цвет | Clear colorless |
| Удельный вес | 0.960 (20/4℃) |
| РН | 3.94(1 mM solution);3.42(10 mM solution);2.92(100 mM solution); |
| Запах | at 1.00 % in dipropylene glycol. sharp acetic cheese butter fruit |
| Odor Type | cheesy |
| Биологические источники | synthetic |
| Порог?обнаружения?запаха? | 0.00019ppm |
| Пределы взрываемости | 2-12.3%(V) |
| Растворимость в воде | MISCIBLE |
| Мерк | 14,1593 |
| Номер JECFA | 87 |
| БРН | 906770 |
| Диэлектрическая постоянная | 3.0(Ambient) |
| Стабильность | Flammable. Incompatible with strong oxidizing agents, aluminium and most other common metals, alkalies, reducing agents. |
| ИнЧИКей | FERIUCNNQQJTOY-UHFFFAOYSA-N |
| LogP | 1.1 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | BUTYRIC ACID |
| FDA 21 CFR | 182.63 |
| Справочник по базе данных CAS | 107-92-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 40UIR9Q29H |
| Справочник по химии NIST | Butanoic acid(107-92-6) |
| Система регистрации веществ EPA | Butyric acid (107-92-6) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | C,Xi | |||||||||
| Заявления о рисках | 34 | |||||||||
| Заявления о безопасности | 26-36-45 | |||||||||
| РИДАДР | UN 2820 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ES5425000 | |||||||||
| F | 13 | |||||||||
| Температура самовоспламенения | 824 °F | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2915 60 19 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 107-92-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 8.79 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Масляная кислота химические свойства, назначение, производство
Описание
Butyric acid is a carboxylic acid also classified as a fatty acid. It exists in two isomeric forms as shown previously, but this entry focuses on n-butyric acid or butanoic acid. It is a colorless, viscous, rancid-smelling liquid that is present as esters in animal fats and plant oils. Butyric acid exists as a glyceride in butter, with a concentration of about 4%; dairy and egg products are a primary source of butyric acid. When butter or other food products go rancid, free butyric acid is liberated by hydrolysis, producing the rancid smell. It also occurs in animal fat and plant oils.Химические свойства
Butyric acid, C3H7COOH, a colorless liquid with an obnoxious odor, occurring in spoiled butter.It miscible with water, alcohol, and ether.It is used in the synthesis of butyrate ester perfume and flavor ingredients and in disinfectants and pharmaceuticals,Вхождение
Normally occurs in butter as a glyceride. It has been reported found in the essential oils of citronella Ceylon, Eucalyptus globules, Araucaria cunninghamii, Lippia scaberrima, Monarda fistulosa, cajeput, Heracleum giganteum, lavender, Hedeoma pulegioides, valerian, nutmeg, hops, Pastinaca sativa, Spanish anise and others. It has been identified in strawberry aroma, apricot, American cranberry, sour cherry, black currants, butter, milk, strawberry jam, cheeses (blue, cheddar, feta, Swiss, Camembert and romano), raspberry, papaya, coffee mutton, beer, rum, bourbon whiskey and cider.История
Butyric acid gets its name from the Latin butyrum, or butter. It was discovered by Adolf Lieben (1836–1914) and Antonio Rossi in 1869. Butyric acid is one of the simplest fatty acids.Использование
Butyric Acid is a fatty acid that is commonly obtained from butter fat. it has an objectionable odor which limits its uses as a food acid- ulant or antimycotic. it is an important chemical reactant in the manufacture of synthetic flavoring, shortening, and other edible food additives. in butter fat, the liberation of butyric acid which occurs during hydrolytic rancidity makes the butter fat unusable. it is used in soy milk-type drinks and candies.Определение
ChEBI: A straight-chain saturated fatty acid that is butane in which one of the terminal methyl groups has been oxidised to a carboxy group.Методы производства
Butyric acid is produced by oxidation of butyraldehyde (CH3(CH2)2CHO) or butanol (C4H9OH). It can also be formed biologically by the oxidation of sugar and starches using bacteria.Подготовка
Obtained by fermentation of starches and molasses with selective enzymes (Granulo saccharobutyricum); it is subsequently isolated as the calcium salt.Общее описание
A colorless liquid with a penetrating and unpleasant odor. Flash point 170°F. Corrosive to metals and tissue. Density 8.0 lb /gal.Реакции воздуха и воды
Water soluble.Профиль реактивности
(3R,4S)-1-Benzoyl-3-(1-methoxy-1-methylethoxy)-4-phenyl-2-azetidinone can react with oxidizing agents. Incandescent reactions occur with chromium trioxide above 212°F. Also incompatible with bases and reducing agents. May attack aluminum and other light metals .Опасность
Strong irritant to skin and tissue.Угроза здоровью
Inhalation causes irritation of mucous membrane and respiratory tract; may cause nausea and vomiting. Ingestion causes irritation of mouth and stomach. Contact with eyes may cause serious injury. Contact with skin may cause burns; chemical is readily absorbed through the skin and may cause damage by this route.Пожароопасность
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Приложение биотехнологий?
Butyrate is produced as end - product of a fermentation process solely performed by obligate anaerobic bacteria. Fermented Kombucha "tea" includes butyric acid as a result of the fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861.The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is then oxidized into acetyl coenzyme A using a unique mechanism that involves an enzyme system called pyruvate - ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of elemental hydrogen (H2) are formed as waste products from the cell.
Профиль безопасности
Moderately toxic by ingestion, skin contact, subcutaneous, intraperitoneal, and intravenous routes. Human mutation data reported. Severe skin and eye irritant. A corrosive material. Combustible liquid. Could react with oxidizing materials. Incandescent reaction with chromium trioxide above 100'. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.Безопасность
The United States Environmental Protection Agency rates and regulates butyric acid as a toxic substance.Personal protective equipment such as rubber or PVC gloves, protective eye goggles, and chemical-resistant clothing and shoes are used to minimize risks when handling butyric acid.
Inhalation of butyric acid may result in soreness of throat, coughing, a burning sensation and laboured breathing. Ingestion of the acid may result in abdominal pain, shock, and collapse. Physical exposure to the acid may result in pain, blistering and skin burns, while exposure to the eyes may result in pain, severe deep burns and loss of vision.
Возможный контакт
In manufacture of butyrate esters, some of which go into artificial flavoring. Incompatibilities: May form explosive mixture with air. Incompatible with sulfuric acid, caustics, ammonia, aliphatic amines; isocyanates, strong oxidizers; alkylene oxides; epichlorohydrinПеревозки
UN2820 Butyric acid, Hazard class: 8; Labels: 8—Corrosive material. UN2529 Isobutyric acid, Hazard Class: 3; Labels: 3—Flammable liquid, 8—Corrosive materialМетоды очистки
Distil the acid, them mix it with KMnO4 (20g/L), and fractionally redistil, discarding the first third of the distillate [Vogel J Chem Soc 1814 1948]. [Beilstein 2 IV 779.]Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Масляная кислота запасные части и сырье
Масляная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +8615866703830 | China | 8497 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 |