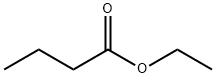
ЭТИЛБУТИРАТ
- английское имяEthyl butyrate
- CAS №105-54-4
- CBNumberCB1852659
- ФормулаC6H12O2
- мольный вес116.16
- EINECS203-306-4
- номер MDLMFCD00009394
- файл Mol105-54-4.mol
химическое свойство
| Температура плавления | -93 °C (lit.) |
| Температура кипения | 120 °C (lit.) |
| плотность | 0.875 g/mL at 25 °C (lit.) |
| плотность пара | 4 (vs air) |
| давление пара | 15.5 mm Hg ( 25 °C) |
| показатель преломления | n |
| FEMA | 2427 | ETHYL BUTYRATE |
| Fp | 67 °F |
| температура хранения | Store below +30°C. |
| растворимость | Soluble in propylene glycol, paraffin oil, and kerosene. |
| форма | Liquid |
| цвет | Clear colorless |
| Запах | Like apple or pineapple. |
| Порог?обнаружения?запаха? | 0.00004ppm |
| Odor Type | fruity |
| Биологические источники | synthetic |
| Растворимость в воде | practically insoluble |
| Мерк | 14,3775 |
| Номер JECFA | 29 |
| БРН | 506331 |
| Диэлектрическая постоянная | 5.1(20℃) |
| Стабильность | Stable. Flammable. Incompatible with strong oxidizing agents, acids, bases. |
| ИнЧИКей | OBNCKNCVKJNDBV-UHFFFAOYSA-N |
| LogP | 2.433 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | ETHYL BUTYRATE |
| FDA 21 CFR | 182.60 |
| Справочник по базе данных CAS | 105-54-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | UFD2LZ005D |
| Справочник по химии NIST | Butanoic acid, ethyl ester(105-54-4) |
| Система регистрации веществ EPA | Ethyl butyrate (105-54-4) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 10-36/37/38 | |||||||||
| Заявления о безопасности | 16-26-36 | |||||||||
| РИДАДР | UN 1180 3/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ET1660000 | |||||||||
| Температура самовоспламенения | 865 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29156000 | |||||||||
| Банк данных об опасных веществах | 105-54-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 13,050 mg/kg (Jenner) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
ЭТИЛБУТИРАТ химические свойства, назначение, производство
Описание
Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH3CH2CH2COO.CH2CH3.It is soluble in propylene glycol, paraffin oil, and kerosene.It has a fruity odor, similar to pineapple.Химические свойства
Ethyl butyrate is a colorless liquid. Pineapple odor. The Odor Threshold is 0.015 ppm.Вхождение
Identified by gas chromatography in olive oil and other vegetable oils. Reported found in apple, banana, citrus peel oils and juices, cranberry, blueberry, black currants, guava, grapes, papaya, strawberry, onion, leek, cheeses, chicken, beef, beer, cognac, rum, whiskies, cider, sherry, grape wines, coffee, honey, soybeans, olives, passion fruit, plums, mushroom, mango, fruit brandies, kiwifruit, mussels and pawpaw.Использование
It is commonly used as artificial flavoring resembling orange juice or pineapple in alcoholic beverages (e.g. martinis, daiquiris etc.), as a solvent in perfumery products, and as a plasticizer for cellulose. In addition, ethyl butyrate is often also added to orange juice, as most associate its odor with that of fresh orange juice.Ethyl butyrate is one of the most common chemicals used in flavors and fragrances. It can be used in a variety of flavors: orange (most common), cherry, pineapple, mango, guava, bubblegum, peach, apricot, fig, and plum. In industrial use, it is also one of the cheapest chemicals, which only adds to its popularity.
Подготовка
By esterification of n-butyric acid with ethyl alcohol in the presence of Twichell’s reagent or MgCI2; also by heating n-butyl alcohol and ethanol over CuO + UO3 catalyst at 270°CМетоды производства
Ethyl Butyrate can be synthesized by reacting ethanol and butyric acid. This is a condensation reaction, meaning water is produced in the reaction as a byproduct.Общее описание
A clear colorless liquid with a pineapple-like odor. Flash point 78°F. Less dense than water and insoluble in water. Vapors heavier than air.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
Ethyl butyrate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. May attack some forms of plastics [USCG, 1999].Опасность
Irritant to eyes and mucous membranes, narcotic in high concentration. Flammable, dangerous fire risk.Угроза здоровью
Inhalation or ingestion causes headache, dizziness, nausea, vomiting, and narcosis. Contact with liquid irritates eyes.Пожароопасность
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back. Containers may explode in fire.Химическая реактивность
Reactivity with Water: No reaction; Reactivity with Common Materials: May attack some forms of plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Профиль безопасности
Wdly toxic by ingestion. A skin irritant. Flammable liquid when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.Возможный контакт
Ethyl butyrate, and aldehyde, is used in flavorings, extracts, perfumery, and as a solvent.Канцерогенность
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.Перевозки
UN1180; UN1178 Ethyl butyrate, Hazard Class: 3; Labels: 3-Flammable liquidМетоды очистки
Dry the ester with anhydrous CuSO4 and distil it under dry nitrogen. [Beilstein 2 IV 787.]Несовместимости
Vapor explosive mixture with air. Ethers can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert. Incompatible with alkaline materials, strong acids, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from strong bases and heatУтилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observedЭТИЛБУТИРАТ запасные части и сырье
ЭТИЛБУТИРАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-15981848961 +86-15981848961 |
China | 497 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| 008657128800458; +8615858145714 |
China | 7738 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 | ||
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | ||
| 86-13657291602 | CHINA | 22963 | 58 |