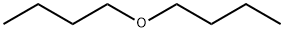
Ди-н-бутиловый эфир
- английское имяDi-n-butyl ether
- CAS №142-96-1
- CBNumberCB2775063
- ФормулаC8H18O
- мольный вес130.23
- EINECS205-575-3
- номер MDLMFCD00009461
- файл Mol142-96-1.mol
| Температура плавления | -98 °C (lit.) |
| Температура кипения | 142-143 °C (lit.) |
| плотность | 0.764 g/mL at 25 °C (lit.) |
| плотность пара | 4.48 (vs air) |
| давление пара | 4.8 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 77 °F |
| температура хранения | Store at <= 20°C. |
| растворимость | H2O: soluble0.113g/L at 20°C |
| форма | Liquid |
| цвет | Clear colorless |
| РН | 5.2 (0.2g/l, H2O, 20℃) |
| Относительная полярность | 1.7 |
| Запах | Mild, ether-like. |
| Водородный показатель | 5.2 |
| Пределы взрываемости | 0.9-8.5%(V) |
| Относительная плотность газов(воздух=1) | 0.764 g/mL at 25 °C |
| Растворимость в воде | 0.03 g/100 mL (20 ºC) |
| Мерк | 14,1569 |
| БРН | 1732752 |
| Диэлектрическая постоянная | 3.1(25℃) |
| Стабильность | Stable. Flammable. May form peroxides in storage. Incompatible with strong oxidizing agents. |
| ИнЧИКей | DURPTKYDGMDSBL-UHFFFAOYSA-N |
| LogP | 3.35 |
| Справочник по базе данных CAS | 142-96-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | PBM2R52P5G |
| Справочник по химии NIST | n-Butyl ether(142-96-1) |
| Система регистрации веществ EPA | Butyl ether (142-96-1) |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 10-36/37/38-52/53 | |||||||||
| Заявления о безопасности | 61-24/25 | |||||||||
| РИДАДР | UN 1149 3/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | EK5425000 | |||||||||
| Температура самовоспламенения | 365 °F | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29091990 | |||||||||
| Банк данных об опасных веществах | 142-96-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 7.4 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P273:Избегать попадания в окружающую среду.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Ди-н-бутиловый эфир химические свойства, назначение, производство
Описание
Butyl ether (dibutyl ether) is a colorless, stable liquid, with a mild ether-like odor. It is immiscible with water, with a specific gravity of 0.8, which is lighter than water. Butyl ether is a moderate fire risk and will form explosive peroxides on aging. Flammable range is 1.5%–7.6% in air, with a boiling point of 286°F (141°C) and a flash point of 77°F (25°C). Ignition temperature is 382°F (194°C), and the vapor density is 4.5, which is heavier than air. In addition to flammability, butyl ether is toxic on prolonged inhalation. The four-digit UN identification number is 1149. The NFPA 704 designation is health 2, flammability 3, and reactivity 1. The primary use is as a solvent.Химические свойства
Di-n-butyl ether is colourless liquid with ether-like odourИспользование
Di-n-butyl ether is used as a solvent for Grignard, Witting and alkyl lithium reactions. It is also used as a solvent for oils and fats and some natural and synthetic resins. It is considered as a replacement for terathydofuran in organic synthesis due to its less water and peroxide and high boiling point. In the pharmaceutical industry, it is used in the manufacturing process of active pharmaceutical ingredient such as procarbazine and cefaclor. In addition to this, it is used as an important solvent for the application of the coating.Общее описание
Di-n-butyl ether is a clear colorless liquid with an ethereal odor. Flash point below 141°F. Less dense than water and insoluble in water. Vapors heavier than air. Irritates the eyes, nose, throat, and respiratory tract.Реакции воздуха и воды
Highly flammable. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979 p.151-154, 164]. A mixture of liquid air and diethyl ether exploded spontaneously [MCA Case History 616 1960]. Insoluble in water.Профиль реактивности
Ethers, such as BUTYL ETHER can act as bases. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.Опасность
Toxic on prolonged inhalation. Flammable, moderate fire risk. May form explosive peroxides, especially in anhydrous form.Угроза здоровью
Inhalation causes irritation of nose and throat. Liquid irritates eyes and may irritate skin on prolonged contact. Ingestion causes irritation of mouth and stomach.Пожароопасность
Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Промышленное использование
n-Butyl ether is used in synthesis reactions that require an anhydrous, inert solvent. This ether is a valuable extraction solvent for aqueous solutions because of its low water solubility. n-Butyl ether when mixed with ethanol or butanol is an excellent solvent for ethyl cellulose.Профиль безопасности
Mildly toxic by inhalation, ingestion, and skin contact. Human systemic effects by inhalation: conjunctiva irritation and unspecified nasal effects. An experimental skin and human eye irritant. See also ETHERS. Dangerous fire hazard when exposed to heat, flame, oroxidizers. Incompatible with NCL and oxidizing materials. To fight fire, use alcohol foam, dry chemical. When heated to decomposition it emits acrid smoke and fumes.Возможный контакт
Di-n-butyl ether is used as a solvent for hydrocarbons, fatty materials; extracting agent in used metals separation; solvent purification, making other chemicals. Incompatibilities: May form explosive mixture with air. May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with strong acids; oxidizers. Contact with air or light may form unstable and explosive peroxides, especially anhydrous form.Экологическая судьба
Butyl ether has the ability to dissolve lipids. As a result, it causes irritation and pain on contact with the eyes and nasal mucosa. It also causes dermal irritation and dermatitis on contact with the skin. Damage caused by butyl ether appears to be scattered loss of epithelial cells due to solution of phospholipid cell membranes. At the central nervous system (CNS) level, butyl ether, like other volatile organic solvents, depresses the CNS by dissolving in the lipid membrane of the cells and disrupting the lipid matrix. These effects are known as membrane fluidization. At the molecular level, membrane fluidization disrupts solute gradient homeostasis, which is essential for cell function.Перевозки
UN1149 Butyl ethers & Dibutyl ethers, Hazard Class: 3; Labels: 3—Flammable liquidМетоды очистки
Peroxides (detected by the liberation of iodine from weakly acid HCl solutions of 2% KI) can be removed by shaking 1L of ether with 5-10mL of a solution comprising of 6.0g of ferrous sulfate and 6mL conc H2SO4 and 110mL of water, with aqueous Na2SO3, or with acidified NaI, water, then aqueous Na2S2O3. After washing with dilute NaOH, KOH, or Na2CO3, then water, the ether is dried with CaCl2 and distilled. It can be further dried by distillation from CaH2 or Na (after drying with P2O5), and stored in the dark with Na or NaH. The ether can also be purified by treating with CS2 and NaOH, expelling the excess sulfide by heating. The ether is then washed with water, dried with NaOH and distilled [Kusama & Koike J Chem Soc Jpn, Pure Chem Sect 72 229 1951]. Other purification procedures include passage through an activated alumina column to remove peroxides, or through a column of silica gel, and distillation after adding about 3% (v/v) of a 1M solution of MeMgI in n-butyl ether. [Beilstein 1 IV 1520.]Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Ди-н-бутиловый эфир запасные части и сырье
сырьё
1of2
запасной предмет
- Butyldi-1-adamantylphosphine
- 7-хлорхинолин-6-Амин
- 2-CHLOROQUINOLIN-6-AMINE
- 3-CHLORO-6-NITROQUINOLINE
- Butyl glycidyl ether
- potassium ω-hydroperfluroheptylate
- 8-хлорхинолин-6-Амин
- 6-(TERT-BUTYL)PYRIMIDINE-2,4-DIAMINE
- 5-хлорхинолин-6-Амин
- N-n-Butyl benzene sulfonamide
1of4
Ди-н-бутиловый эфир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12495 | 58 | |
| +8617531190177 | China | 5873 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20290 | 58 | |
| 571-85586718 +8613336195806 |
China | 29798 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21663 | 55 | |
| 025-83697070 | CHINA | 3012 | 60 | |
| +undefined-21-51877795 | China | 32836 | 60 | |
| +86-0551-65418679 +8618949832763 |
China | 2989 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29888 | 58 | |
| +86-19930503282 | China | 8820 | 58 |