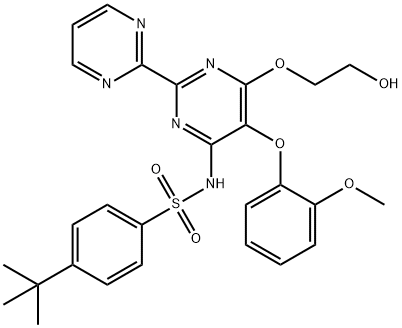
Бозентан
- английское имяBosentan
- CAS №147536-97-8
- CBNumberCB3425624
- ФормулаC27H29N5O6S
- мольный вес551.61
- EINECS643-099-1
- номер MDLMFCD00867375
- файл Mol147536-97-8.mol
химическое свойство
| Температура плавления | 107-110°C |
| Температура кипения | 742.3±70.0 °C(Predicted) |
| плотность | 1.325±0.06 g/cm3(Predicted) |
| температура хранения | -20°C Freezer |
| растворимость | DMSO (Slightly), Methanol (Slightly) |
| форма | Solid |
| пка | 4.01±0.10(Predicted) |
| цвет | White to Pale Yelloow |
| Справочник по базе данных CAS | 147536-97-8(CAS DataBase Reference) |
| FDA UNII | XUL93R30K2 |
| Код УВД | C02KX01 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P281:Пользоваться надлежащим индивидуальным защитным снаряжением.
Бозентан химические свойства, назначение, производство
Химические свойства
Pale Yellow to Off-White SolidИспользование
A mixed endothelin receptor antagonist. Used as a vasodilator. Antihypertensive.Общее описание
Bosentan, N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-pyrimidin-2-yl-pyrimidin-4-yl]-4-tertbutyl-benzenesulfonamide (Tracleer, Bozentan), was thefirst endothelin receptor antagonist marketed in the UnitedStates. Bosentan works by competitively blocking the endothelinreceptor subtypes ETA and ETB. In binding to thereceptors, it blocks the effects of endothelin, which includeconstriction of the vascular smooth muscle, which leads tonarrowing of the blood vessels and hypertension. Althoughit is not selective for the ETA receptors, it does have a higheraffinity for that subtype over ETB. However, the clinical significanceof selectivity over preferential receptor bindinghas not been demonstrated. Bosentan is an inducer ofCYP2C9 and CYP3A4, and patients using bosentan must bemonitored for liver toxicity.Опасность
A reproductive hazard.Фармакокине?тика
Bosentan is mainly eliminated from the body by hepatic metabolism and subsequent biliary excretion of the metabolites. Three metabolites have been identified, formed by CYP2C9 and CYP3A4. The pharmacokinetics of bosentan are dose-proportional up to 500 mg/day (multiple doses). The pharmacokinetics of bosentan in pediatric patients with PAH are comparable to those in healthy subjects, whereas adult patients with PAH show a twofold increase in clearance. Severe renal impairment and mild hepatic impairment do not have a clinically relevant influence on its pharmacokinetics. Bosentan generally should be avoided in patients with moderate or severe hepatic impairment and/or elevated liver aminotransferases. Inhibitors of CYP3A4 increase the plasma concentration of bosentan as well as cause an increase in the clearance of drugs metabolized by CYP3A4 and CYP2C9 because of induction of these metabolizing enzymes. The possibility of reduced efficacy of CYP2C9 and CYP3A4 substrates coadministered with bosentan is increased. No clinically relevant interaction was detected for P-glycoprotein. Bosentan can increase plasma levels of ET-1.Клиническое использование
Bosentan is an orally administered, nonselective ET-1 receptor antagonist blocking ETA and ETB receptors and is approved for the treatment of patients with PAH. Following oral administration, bosentan attains peak plasma concentrations in approximately 3 hours, with an absolute bioavailability of approximately 50%. Food has no clinically relevant effect on its absorption recommended doses. Bosentan is approximately 98% bound to albumin, with a volume of distribution of 30 L. Its terminal half-life after oral administration is 5.4 hours and is unchanged at steady state.Побочные эффекты
Adverse effects include hypotension, headache, flushing, increased liver aminotransferases, leg edema, and anemia. Bosentan may cause birth defects and, therefore, is contraindicated in pregnancy. It also can cause liver injury.Бозентан запасные части и сырье
Бозентан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| 28-85305008 | CHINA | 964 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +8613367258412 | China | 10319 | 58 |