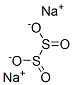
Дитионит натрия
- английское имяSodium dithionite
- CAS №7775-14-6
- CBNumberCB1155576
- ФормулаNa2O4S2
- мольный вес174.11
- EINECS231-890-0
- номер MDLMFCD00011640
- файл Mol7775-14-6.mol
| Температура плавления | 300 °C |
| Температура кипения | 1390°C |
| плотность | 2.13 |
| Плотность накопления | 1250kg/m3 |
| Fp | >100°C |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | 250 g/L (20°C) |
| форма | Powder/Solid |
| цвет | White |
| Запах | None or slight scent of sulfur dioxide |
| РН | 5.5-8.5 (50g/l, H2O, 20℃) |
| Растворимость в воде | 250 g/L (20 ºC) |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,8626 |
| Стабильность | Stable, but air sensitive. Incompatible with strong acids, strong oxidizing agents, water, moisture. |
| LogP | -2.756 (est) |
| Справочник по базе данных CAS | 7775-14-6(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM HYDROSULFITE |
| Специальный комитет по веществам GRAS | Sodium hydrosulfite (packaging) |
| FDA 21 CFR | 177.2800; 182.90 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 2K5B8F6ES1 |
| Система регистрации веществ EPA | Sodium hydrosulfite (7775-14-6) |
| UNSPSC Code | 12352302 |
| NACRES | NA.21 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 7-22-31 | |||||||||
| Заявления о безопасности | 26-28-43-7/8-43E-28A | |||||||||
| РИДАДР | UN 1384 4.2/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| F | 1-10 | |||||||||
| Температура самовоспламенения | >200 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.2 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28311010 | |||||||||
| Банк данных об опасных веществах | 7775-14-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2500 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H251:Возможно самопроизвольное возгорание.
-
оператор предупредительных мер
P235:Держать в прохладном месте.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Дитионит натрия химические свойства, назначение, производство
Химические свойства
White solidИспользование
Sodium dithionite is a reducing agent for vat dyes,reducing hair bleaching agents,vat dyes printing auxiliaries, silk scouring and bleaching agents, coloring agents and objects stripping vat cleaning agent, particularly in dyeing with indigo and vat dyes; bleaching soaps, straw; removing dyes from dyed fabrics.Определение
ChEBI: An inorganic sodium salt that is the disodium salt of dithionous acid.Методы производства
An alternative route for dithionite production is the reduction of sodium bisulfite with sodium borohydride. Sodium borohydride is obtained by reacting boron trimethyl ester, B(OCH3)3, with sodium hydride, NaH. The resulting product is hydrolyzed with water, and methanol is evaporated. An alkaline, aqueous solution is obtained, containing about 12% NaBH4 and 40% NaOH. This solution is commercially available. Reaction to dithionite is made on-site by adding sulfur dioxide and some additional caustic soda. Storages, handling and mixing of sulfur dioxide and of the Borol® liquid are not everywhere cost competitive.NaBH4+8NaOH+8SO2 → 4Na2S2O4+NaBO2+6H2O.
Подготовка
Sodium dithionite is today produced mainly from sodium formate and sulfur dioxide. Zinc dithionite, prepared on-site from sulfur dioxide and zinc dust, was formerly more important than the sodium salt. The heavy metal zinc ended up in the effluent, which caused environmental problems. Today in pulp mills on-site production of zinc dithionite is no longer practiced. The number of plants using sodium amalgam and sulfur dioxide for dithionite production is decreasing with the number of amalgam cells in electrolysis. On-site generation of dithionite from alkaline sodium borohydride solution, sodium bisulfite, and sulfur dioxide is used by some mills.Sodium dithionite is available as crystalline powder (&90% Na2S2O4) or as a refrigerated 150 g/L solution stabilized with alkali. The white crystals can decompose on heating. Low alkalinity, pH8 to 13, stabilizes dithionite solution at low temperature.
These solutions should be kept well below 10 °C with the exclusion of air. In presence of air, oxidation rapidly yields sulfate and sulfite.
The powder product prepared via the amalgam route dissolves into an alkaline solution as it contains sodium carbonate and sulfite. This can trigger the precipitation of calcium carbonate (water hardness) in the solution tank. Therefore, it is recommended to add small amounts of chelants during the dissolution step. (Chelants are not required to stabilize the bleaching reaction.) Sodium dithionite prepared by the formate route dissolves into a slightly acidic solution. Dithionite powder can ignite if exposed to high humidity or elevated temperature.
Общее описание
Sodium dithionite is a whitish to light yellow crystalline solid having a sulfur dioxide-like odor. Sodium dithionite spontaneously heats on contact with air and moisture. This heat may be sufficient to ignite surrounding combustible materials. Sodium dithionite is soluble in water. Under prolonged exposure to fire or intense heat containers of Sodium dithionite may violently rupture. Sodium dithionite is used in dyeing and to bleach paper pulp.Профиль реактивности
Inorganic reducing agents, such as Sodium dithionite, react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Their reactions with oxidizing agents may be violent. Sulfites and hydrosulfites (dithionites) can react explosively with strong oxidizing agents (sodium chlorite). Sulfites generate gaseous sulfur dioxide in contact with oxidizing acids and nonoxidizing acids.Опасность
Fire risk in contact with moisture. To extin- guish fires, flood the reacting mass with water.Угроза здоровью
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.Пожароопасность
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.Профиль безопасности
Toxic and an irritant. An allergen. Flammable when exposed to heat or flame. Ignites on contact with water or sodium chlorite. To extinpsh fires, flood the reacting mass with water. Decomposes violently when heated to 19OOC and emits toxic fumes of SOx and NazOхранилище
Sodium dithionite crystals are available in steel containers (1 or 2 tons) or in steel drums (200 kg). Because of the danger of spontaneous ignition in humid air, sodium dithionite must be stored under dry and cool conditions. The sites for the preparation of dithionite solutions must permit handling without risks, high humidity should be avoided and remote fire control should be available.Commercial dithionite products are classified as self-igniting hazardous goods (Class 4.2, UN 1384). Local rules for transportation and storage must be obeyed.
Дитионит натрия запасные части и сырье
сырьё
1of3
запасной предмет
- SODIUM DITHIONATE
- Vitamin B2
- 8-метилксантиновый
- 4-аминобензилового спирта
- 4-AMINORESORCINOL HYDROCHLORIDE
- 4.4'-ДИАМИНОДИФЕНИЛАМИНСУЛЬФАТ
- 5-фториндол-2-карбоновая кислота
- Натрия салицилат
- 3-аминофталгидразид
- (2,4-ДИАМИНОПТЕРИДИН-6-YL) МЕТАНОЛА ГИДРОХЛОРИД ГИДРАТ
- 4-(1,2,3-ТИАДИАЗОЛ-4-ИЛ)АНИЛИН
- 4-Азаиндол
- 5,6-DIAMINO-2,4-DIHYDROXYPYRIMIDINE SULFATE
- TRIFLUOROMETHYL SULFINYL CHLORIDE
- 1H-1,2,3-Triazolo[4,5-d]pyrimidin-7-amine
- 4,5,6-Триаминопиримидин сульфат
- Глиоксальбис(2-гидроксианил)
- Рибофлавин
- 8-BROMO-3-METHYL-3,7-DIHYDRO-PURINE-2,6-DIONE
- 5-гидроксиантраниловая кислота
- 8-AZAGUANINE
- N-(2,5-Dimethoxyphenyl)-2-hydroxydibenzofuran-3-carboxamide
- 3-метилксантин
- 3-(3-АМИНОФЕНИЛ)-2H-ХРОМЕН-2-ОН
- (2-CYANO-3-PYRIDINYL)CARBAMIC ACID, 1,1-DIMETHYL ETHYL ESTER
1of8
Дитионит натрия поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-57-80696996 +86-19816093969 |
China | 16 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7782 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |