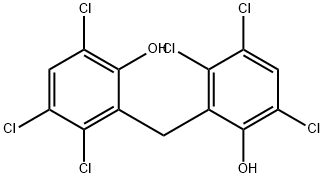
Гексахлорофен
- английское имяHexachlorophene
- CAS №70-30-4
- CBNumberCB8687902
- ФормулаC13H6Cl6O2
- мольный вес406.9
- EINECS200-733-8
- номер MDLMFCD00002171
- файл Mol70-30-4.mol
| Температура плавления | 163-165 °C(lit.) |
| Температура кипения | 519.9°C (rough estimate) |
| плотность | 1.6065 (rough estimate) |
| показатель преломления | 1.5550 (estimate) |
| Fp | 11 °C |
| температура хранения | 2-8°C |
| растворимость | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; Ethanol:PBS (pH 7.2) (1:4): 0.2 mg/ml |
| форма | crystalline |
| пка | pKa 4.89 ± 0.02(H2O,t = 25.0±0.1,I=0.1(NaCl)) (Uncertain) |
| цвет | off-white to tan |
| Растворимость в воде | 19mg/L(25 ºC) |
| λмакс | 300nm(MeOH)(lit.) |
| Мерк | 14,4680 |
| БРН | 2064407 |
| Стабильность | Stable. Incompatible with alkalies, akaline earths, tweens, strong oxidizers. |
| Справочник по базе данных CAS | 70-30-4(CAS DataBase Reference) |
| FDA 21 CFR | 310.545 |
| Рейтинг продуктов питания EWG | 4-8 |
| FDA UNII | IWW5FV6NK2 |
| Код УВД | D08AE01 |
| МАИР | 3 (Vol. 20, Sup 7) 1987 |
| Справочник по химии NIST | 2,2'-Methylenebis(3,4,6-trichlorophenol)(70-30-4) |
| Система регистрации веществ EPA | Hexachlorophene (70-30-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,N,F | |||||||||
| Заявления о рисках | 24/25-50/53-52/53-39/23/24/25-23/24/25-11-51/53 | |||||||||
| Заявления о безопасности | 20-37-45-60-61-36/37-16 | |||||||||
| РИДАДР | UN 2875 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | SM0700000 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29072990 | |||||||||
| Банк данных об опасных веществах | 70-30-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in adult male, female rats (mg/kg): 66, 57 orally (Gaines, Linder) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H301+H311:Токсично при проглатывании или при контакте с кожей.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
Гексахлорофен химические свойства, назначение, производство
Описание
Hexachlorophene (HCP) is a chlorinated bisphenol antiseptic and was introduced for use as an antibacterial component in drug and cosmetic products in 1941. It has bacteriostatic activity against gram-positive organisms (e.g., staphylococcus) but not against gram-negative organisms. HCP is readily absorbed orally and through the skin of humans, especially skin of premature infants or damaged skin. Hexachlorophene is a neurotoxicant, and symptomology may include lethargy, muscle weakness, irritability, cerebral edema, and paralysis leading to coma and death. The US Food and Drug Administration restricted the use of hexachlorophene preparation to ≤0.1% in 1972 and approved it for surgical scrubbing and handwashing. HCP is no longer used extensively in hospitals, rest homes, etc., because of its toxicity.Химические свойства
Hexachlorophene is a crystalline solid com pound.Использование
Hexachlorophene is a topical antiseptic in germicidal soaps, creams, deodorants, cleansers, shampoos, after-shave creams, pHisoHex surgical cleanser and in veterinary medicine.Показания
Hexachlorophene is a bacteriostatic antiseptic effective against gram-positive organisms, specifically Staphylococcus. It has a cumulative and sustained effect. Although it has a slow onset of action, with repeated use a film develops on the skin that has long-acting properties. It is not effective against gram-negative organisms or yeast. It is postulated to affect bacterial electron transport and membrane function. With repeated use, it penetrates through the stratum corneum.It is often used for preoperative surgical scrubbing. One retrospective study showed that it may be teratogenic if used excessively by pregnant women, although there have been no subsequent, well-controlled studies that substantiate this. It should not be used in infants, particularly those who are premature or have dermatoses. It is toxic to tissues when applied to open wounds.
Определение
ChEBI: An organochlorine compound that is diphenylmethane in which each of the phenyl groups is substituted by chlorines at positions 2, 3, and 5, and by a hydroxy group at position 6. An antiseptic that is effective against Gram-positive organisms, it is used in soaps and creams for the treatment of various skin disorders. It is also used in agriculture as an acaricide and fungicide, but is not approved for such use within the European Union.Подготовка
Hexachlorophene is formed by reaction of 2,4,5-trichlorophenol with paraformaldehyde in fuming sulfuric acid (20% SO3) at 65–135 ℃. Hexachlorophene is a strong bacteriostat which also has a microbicidal effect at high concentrations. The use of Hexachlorophene as an antimicrobial agent in cosmetics, medicinal soaps, detergent solutions, and textiles has, however, been discontinued because of its toxicity.Всемирная организация здравоохранения(ВОЗ)
Hexachlorophene, an antimicrobial agent, was introduced in 1948 in proprietary liquid preparations and powders and was subsequently used extensively as a topical antiseptic. By the early 1970s its use in infants had been conclusively demonstrated to cause encephalopathy as a result of transdermal absorption. More recently it has been suggested that the drug has a teratogenic potential. Many regulatory authorities have placed rigorous restrictions on the medicinal use of hexachlorophene, particularly in preparations intended for infants. However, its use still commonly remains permissible at low concentrations as a preservative in toiletries and cosmetics. (Reference: (WHODI) WHO Drug Information, 3, 6, 1978)Общее описание
A white free-flowing odorless powder. Insoluble in water and denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Hexachlorophene is incompatible with strong oxidizers. Hexachlorophene forms salts with alkalis and alkaline earths.Опасность
FDA prohibits use unless prescribed by a physician. Questionable carcinogen.Угроза здоровью
Inhalation of dust is poisonous; irritating to mucous membranes. Eye and skin irritant. Poisonous if swallowed. Symptoms following ingestion include anorexia, nausea, vomiting, abdominal cramps, and diarrhea. Dehydration may be severe and may be associated with shock.Возможный контакт
HCP has been used as an antibacterial agent in a wide variety of consumer products, including soaps and deodorants; as a disinfectant. It has also been used as an antifungal agent to treat various citrus fruits and vegetables.Экологическая судьба
HCP adsorbs very strongly to soil and is not expected to leach to groundwater. It may undergo slow photodegradation on the surface of soils and water based on its absorption of light (290 nm). No information is available on its biodegradation in soil or surface water. HCP released in water adsorbs very strongly to sediments and may bioconcentrate in aquatic organisms. It has an estimated bioconcentration factor of 317 000. HCP is not expected to hydrolyze or to significantly evaporate from water. When released into the air, HCP is expected to be mainly in the particle-sorbed state due to its low vapor pressure and high estimated Koc. It is expected to be removed from the atmosphere primarily by dry deposition, but it is also degraded by reaction with photochemically produced hydroxyl radicals, with an estimated vapor phase half-life of 2.5 days.Перевозки
UN2875 Hexachlorophene, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Методы очистки
It forms needles from MeOH, C2H4Cl2, or toluene. The diacetate has m 175-176o (EtOH). A disinfectant is also available in MeOH (5mg/L). [Beilstein 6 III 5407, 6 IV 6659.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Утилизация отходов
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste con taining this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treat ment, and waste disposal.Гексахлорофен запасные части и сырье
Гексахлорофен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86 523-86200360 | CHINA | 358 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-0571-87635730 +8615858229168 |
China | 1478 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 0917-3909592 13892490616 |
China | 9310 | 58 |