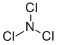
Трихлор нитрид
- английское имяTrichlorine nitride
- CAS №10025-85-1
- CBNumberCB0852223
- ФормулаCl3N
- мольный вес120.37
- EINECS233-045-1
- файл Mol10025-85-1.mol
химическое свойство
| Температура плавления | -27°C |
| Температура кипения | 71°C (estimate) |
| плотность | 1.653 |
| растворимость | insoluble in H2O; soluble in CS2, benzene, ctc 2 |
| пка | -22.67±0.70(Predicted) |
| форма | yellow oily liquid |
| цвет | yellow oily liquid; explodes, explosive |
| Растворимость в воде | insoluble H2O, decomposes in H2O after 24h; soluble CS2, phosphorus trichloride, benzene, CCl4, CHCl3 [MER06] |
| Справочник по базе данных CAS | 10025-85-1 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | VA681HRW8W |
| Система регистрации веществ EPA | Nitrogen trichloride (10025-85-1) |
| РИДАДР | 3099 |
| Класс опасности | 5.1 |
| Группа упаковки | II |
| Банк данных об опасных веществах | 10025-85-1(Hazardous Substances Data) |
Трихлор нитрид химические свойства, назначение, производство
Химические свойства
Yellow oil or rhombic crystals.Insoluble in cold water; decomposes in hot water; soluble in chloroform, phosphorus trichloride, and carbon disulfide.Физические свойства
Yellow, oily, heavy liquid; pungent odor; density 1.653 g/mL; freezes to rhombohedral crystalline solid below -40°C; evaporates in air rapidly; vaporpressure 150 torr at 20°C; explodes when heated at 93°C; highly unstable, decomposes explosively in light; insoluble in water, decomposes slowly in cold water after several hours; decomposes in hot water; soluble in benzene, chloroform, carbon tetrachloride, carbon disulfide and phosphorus trichloride.Использование
Bleaching of flour; wastage control of citrus fruit.Подготовка
Nitrogen trichloride is prepared by passing chlorine gas into slightly acid solution of ammonium chloride. The product is continuously extracted with carbon tetrachloride:NH4Cl + 3Cl2 → NCl3 + 4HCl
Hypochlorous acid, HOCl, also may be used instead of chlorine in such preparation.
Nitrogen trichloride can be prepared by the action of anhydrous chlorine with anhydrous ammonia:
3Cl2 + NH3 → NCl3 + 3HCl
Nitrogen trichloride is made commercially by electrolyzing an acidified solution of ammonium chloride.
Опасность
Explodes when heated to approximately 200F (93C) or when exposed to direct sunlight. Toxic by ingestion and inhalation, strong irritant.Профиль безопасности
Moderately toxic by inhalation. An irritant to the eyes, skin, mucous membranes, and a systemic central nervous system irritant. An explosive sensitive to impact, light, and ultrasound. The solid explodes on melting. The liquid explodes above 60°C. Concentrated solutions are also explosive. Explosive decomposition is initiated by contact with: concentrated ammonia, arsenic, dinitrogen tetraoxide, hydrogen sulfide, hydrogen trisulfide, nitrogen oxide, organic matter, ozone, phosphine, phosphorus, potassium cyanide, potassium hydroxide solutions, selenium, hydrogen chloride, hydrogen fluoride, hydrogen bromide, hydrogen iodide. mxtures with chlorine + hydrogen are potentially explosive. Upon decomposition it emits toxic fumes of Cl- and NOx. See also CHLORIDES.Трихлор нитрид запасные части и сырье
Трихлор нитрид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 021-60549325 18616193163 | China | 4526 | 56 | |
| China | 9484 | 50 | ||
| 029-87576359 15353716720 |
China | 10009 | 58 |