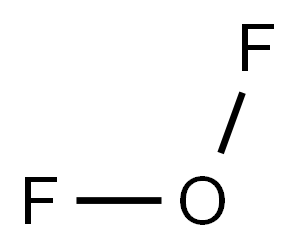
ФТОРИД КИСЛОРОДА
- английское имяOxygen difluoride
- CAS №7783-41-7
- CBNumberCB5851887
- ФормулаF2O
- мольный вес54
- EINECS231-996-7
- файл Mol7783-41-7.mol
| Температура плавления | -223.8° |
| Температура кипения | bp -145.3° |
| плотность | (liq; -224°) 1.90 |
| растворимость | slightly soluble in H2O |
| форма | colorless gas |
| цвет | Colorless gas or yellowish-brown liquid |
| Растворимость в воде | 6.8mL gas/100mL H2O (0°C) [MER06] |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 7BCS2CW398 |
| Система регистрации веществ EPA | Oxygen difluoride (7783-41-7) |
| РИДАДР | 2190 |
| OEL | Ceiling: 0.05 ppm (0.1 mg/m3) |
| Класс опасности | 2.3 |
| Банк данных об опасных веществах | 7783-41-7(Hazardous Substances Data) |
| Токсичность | LC50 (1 hr) inhalation by rats, mice: 2.6, 1.5 ppm (Darmer) |
| ИДЛА | 0.5 ppm |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
H270:Окислитель; может вызвать или усилить возгорание.
-
оператор предупредительных мер
P220:Не допускать соприкосновения с одеждой и другими горючими материалами.
P244:Не допускать попадания жиров и масел в редукционные клапаны.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P370+P376:При пожаре ликвидировать утечку, если это не сопряжено сриском.
P403:Хранить в хорошо вентилируемом месте.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ФТОРИД КИСЛОРОДА химические свойства, назначение, производство
Химические свойства
Oxygen difluoride is a colorless gas. Foul, peculiar odor. Shipped as a nonliquefied compressed gas.Физические свойства
Colorless gas with a characteristic odor; unstable in the presence of moisture, otherwise stable up to 250°C; gas density 2.21g/L at 25°C; liquefies to a yellowish-brown liquid at -144.8°C; density of the liquid 1.90g/ml at -223.8°C; solidifies at -223.8°C; slightly soluble in water, decomposing very slowly; solubility 68ml gas per liter of water at 0°C; slightly soluble in acids and alkali.Использование
Commercial applications of oxygen difluoride are limited. It is used in organic synthesis to prepare fluoropropylenes and acylfluorides. It is used as an oxidizing and fluorinating agent in many preparative reactions and as a monomer in diolefin copolymerization.Подготовка
Oxygen difluoride may be prepared by passing fluorine gas slowly through a dilute solution of caustic soda. Usually a 2% solution of NaOH is suitable for the preparation:2F2 + 2 Na OH → 2NaF + OF2 + H2O
At a higher alkali concentration, oxygen is formed:
2F2 + 4NaOH → 4NaF + 2H2O + O2
Oxygen difluoride can be produced by electrolysis of an aqueous solution of HF or, alternatively, electroylzing molten potassium hydrogen difluoride, KHF2, in the presence of water.
Общее описание
A colorless poisonous gas with a strong peculiar odor. Highly toxic by inhalation. Corrosive to skin and eyes. Can explode on contact with water. Decomposes to toxic gaseous fluorine if heated to high temperature. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Used as an oxidizer for propellants.Реакции воздуха и воды
Violent explosions resulted when a spark was discharged in a 25-70% mixture of Oxygen difluoride with oxygen over water [Mellor 2, Supp. 1:191. 1956].Профиль реактивности
Oxygen difluoride is an oxidizing agent. Mixtures with carbon monoxide, with hydrogen, or with methane explode on sparking [Streng, A. G., Chem. Rev., 1963, 63, p. 610]. May react explosively with adsorbents (silica, alumina, molecular sieves, silica gel) [Streng A. G., Chem. Eng. News, 1965, 43(12), p. 5]; the presence of moisture may render such mixtures shock-sensitive [Metz, F. I., Chem. Eng. News, 1965, 43(7), p. 41]. Gives explosive reactions with diborane, hydrogen sulfide, nitrogen oxide, nitrosyl fluoride, charcoal, sulfur tetrafluoride. Warming of mixtures with halogens, with metal halides, with aluminum chloride, with antimony pentachloride, and with tungsten gives explosions. Ignites on contact with diborane tetrafluoride, phosphorus pentaoxide, red phosphorus, boron, silicon [Bretherick, 5th ed., 1995, p. 1419]. Incompatible with ammonia, arsenic trioxide, chromium trioxide, chlorine in the presence of copper, ozone [Lewis, 3rd ed., 1993, p. 978]. Reacts to incandescence with aluminum, barium, cadmium, magnesium, strontium, zinc, zirconium. Reacts with the alkali metals (lithium, sodium, potassium) [Streng, A. G., Chem. Rev., 1963, 63, p. 611].Опасность
Oxygen difluoride is a highly toxic gas that attacks lungs, manifesting delayed symptoms. It causes irritation of eyes, lungs, and skin. Chronic exposure can lead to pulminary edema and congestion in lungs. Inhalation also can cause systemic toxic effects in humans. LC50 inhalation (rat): 136ppm/1 hr The compound is a very powerful oxidizing agent. Contact with reducing agents can cause explosion.Угроза здоровью
TOXIC; may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution.Пожароопасность
Substance does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.Профиль безопасности
Poison by inhalation. Human systemic effects by inhalation: chronic pulmonary edema or congestion. A corrosive skin, eye, and mucous membrane irritant. Attacks lungs with delayed appearance of symptoms. A very powerful oxidizer. Must be kept away from contact with reducing agents. Explosive reaction with adsorbents (e.g., sllica gel, alumina, molecular sieve), diborane, halogens + heat, metal halides, aluminum chloride, antimony pentachloride (at 1 50℃), tungsten + heat, hydrogen sulfide, liquid nitrogen oxide, nitrosyl fluoride, charcoal, sulfur tetrafluoride. Forms spark-sensitive explosive mixtures with water or combustible gases (e.g., carbon monoxide, hydrogen, methane). Ignites on contact with diborane tetrafluoride, nonmetals (e.g., red phosphorus, boron powder, silicon), phosphorus(V) oxide, nitrogen oxide gas. Incandescent reaction with metals (e.g., aluminum, barium, cadmium, magnesium, strontium, zinc, zirconium, lithium (above 4OO0C)), potassium (above 4OO0C), sodium. Incompatible with NH3, As203, Cl2 + Cu, CrO3, Ir, 03, O2 + H20, Pd, Pt, Rh, Ru, Si02. When heated to decomposition it emits highly toxic fumes of F-. See also FLUORIDESВозможный контакт
Oxygen difluoride is used as an oxidizer in missile propellant systems.Перевозки
Oxygen difluoride, compressed Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Несовместимости
A strong oxidizer. Explodes on contact with steam. Violent reaction with reducing agents; combustible materials; chlorine, bromine, iodine, platinum, metal oxides; moist air; hydrogen sulfide (explosive in ambient air); hydrocarbons, water. Attacks mercury. Reacts, possibly violently, with many materials including porous materials (i.e., alumina, charcoal, and silica), mercury, and phosphorus.Утилизация отходов
Spray or sift on a thick layer of a (1:1) mixture of dry soda ash and slaked lime behind ashield. After mixing, spray water from an atomizer with great precaution. Transfer slowly into a large amount of water. Neutralize and drain into the sewer with sufficient water.