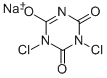
НАТРИЙ дихлоризоцианурат б/в
- английское имяSodium dichloroisocyanurate
- CAS №2893-78-9
- CBNumberCB9181452
- ФормулаC3Cl2N3NaO3
- мольный вес219.95
- EINECS220-767-7
- номер MDLMFCD00006036
- файл Mol2893-78-9.mol
| Температура плавления | 225°C |
| плотность | 1 g/cm3 |
| давление пара | 0.006Pa at 20℃ |
| температура хранения | 0-6°C |
| растворимость | DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated), Water |
| форма | powder to lump |
| цвет | White to Almost white |
| Растворимость в воде | 30g/100ml (25 ºC) |
| Чувствительный | Moisture Sensitive |
| БРН | 4056155 |
| Стабильность | Stable. Oxidizing agent - contact with combustible material may lead to fire. Incompatible with strong bases, strong oxidizing agents. Reacts readily with many nitrogen-containing compounds to form explosive nitrogen triiodide. Moisture-sensitive. |
| InChI | InChI=1S/C3HCl2N3O3.Na/c4-7-1(9)6-2(10)8(5)3(7)11;/h(H,6,9,10);/q;+1/p-1 |
| ИнЧИКей | MSFGZHUJTJBYFA-UHFFFAOYSA-M |
| SMILES | C1(=O)N(Cl)C(=NC(=O)N1Cl)[O-].[Na+] |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | SODIUM DICHLOROISOCYANURATE |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 07M9U9U0LK |
| Система регистрации веществ EPA | Sodium dichloro-s-triazinetrione (2893-78-9) |
| UNSPSC Code | 12352005 |
| NACRES | NA.22 |
| Коды опасности | O,Xn,N,E | |||||||||
| Заявления о рисках | 8-22-31-36/37-50/53-2 | |||||||||
| Заявления о безопасности | 8-26-41-60-61-45 | |||||||||
| РИДАДР | UN 2465 5.1/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | XZ1900000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29336929 | |||||||||
| Банк данных об опасных веществах | 2893-78-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H272:Окислитель; может усилить возгорание.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
НАТРИЙ дихлоризоцианурат б/в химические свойства, назначение, производство
Химические свойства
Dichloroisocyanuric acid, sodium salt, is a white crystalline powder. Chlorine odor. Thermally unstable. It is produced as a result of reaction of cyanuric acid with chlorine. Sodium dichloroisocyanurate is a well established disinfectant used for many purposes including wound cleansing, hospital use, sterilizing babies bottles, disinfection of water for human consumption and disinfection of swimming pools.Использование
Sodium Dichloroisocyanurate (SDIC) widely applied for the sterilization of swimming pool and drinking water, or fighting against infectious diseases, or act as disinfectant in raising silkworm, livestock, poultry and fish. Other applications of SDIC are found in wool shrinkage, textile bleaching, and industrial circulating water cleaning. SDIC is normally supplied in powder and granular, tablets are also available on request. Stabilised chlorine granular (dichlor) are used very widely to chlorinate swimming pool water.Подготовка
Sodium dichloroisocyanurate is produced by chlorination of disodium cyanurate [Na2H(NCO)3] using chlorine (CI2) and neutralization with sodium hydroxide (NaOH).Disodium cyanurate is obtained by action of sodium hydroxide on isocyanuric acid.
Общее описание
White solid with an odor of bleach-like odor. Mixes with water.Реакции воздуха и воды
Water soluble. May vigorously react with water releasing chlorine gas. Material containing less than 39% available chlorine will undergo reactions as described herein, but may take longer to initiate, and the resulting reaction may not be as vigorous [AAR 1992].Профиль реактивности
Contact with ammonium compounds or hydrated salts can cause a very vigorous reaction. Prolonged exposure to heat /fire may result in the vigorous decomposition of the material with the rupture of its containers, Sodium dichloroisocyanurate will accelerate the burning of combustible materials [AAR 1991]. Chlorine plus alcohols would yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991].Опасность
Strong oxidizing material, fire risk near organic materials. Toxic by ingestion.Угроза здоровью
Dust causes sneezing and coughing, moderate irritation of the eyes, and itchiness and redness of the skin. Ingestion causes burns of mouth and stomach.Профиль безопасности
Moderately toxic to humans and animals by ingestion. An experimental teratogen. Experimental reproductive effects. A severe skin and eye irritant. Human systemic effects by ingestion: ulceration or bleeding from stomach. The other main toxic effects were gastrointestinal irritation, salivation, lachrymation, dyspnea, weakness, emaciation, lethargy, diarrhea, coma, and (following very high dosage) death after 1-8 days, with autopsy showing irritation of stomach and gastrointestinal tract, liver dysfunction, and lung congestion. The concentrated material may be a little more toxic, due to greater gastrointestinal irritation. In the dry form, it is not appreciably irritating to dry skin. However, when moist, the concentrated material is irritating to skin, and also may cause severe eye irritation. A powerful oxidizer. Incompatible with combustible materials, ammonium salts, nitrogenous materials. Used to chlorinate swimming pools and in cleaning, bleaching, disinfecting, sanitizing. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and Na2O.Возможный контакт
Dichloroisocyanuric acid salts, are used in cleaning; making dry bleaches, detergents, sanitizers, and disinfectants; in swimming pool and sewage treatment.Перевозки
UN2465 Dichloroisocyanuric acid, dry or Dichloroisocyanuric acid salts, Hazard Class: 5.1; Labels: 5.1-Oxidizer.Несовместимости
A powerful oxidizer. Dust may form explosive mixture with air. Violent reaction with reducing agents; organic matter; easily chlorinated or oxidized materials. Isocyanates are highly flammable and reactive with many compounds, even with themselves. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Reaction with moist air, water or alcohols may form amines and insoluble polyureas and react exothermically, releasing toxic, corrosive or flammable gases, including carbon dioxide; and, at the same time, may generate a violent release of heat increasing the concentration of fumes in the air. Incompatible with amines, aldehydes, alkali metals, ammonia, carboxylic acids, caprolactum, alkaline materials, glycols, ketones, mercaptans, hydrides, organotin catalysts, phenols, strong acids, strong bases, strong reducing agents such as hydrides, urethanes, ureas. Elevated temperatures or contact with acids, bases, tertiary amines, and acyl-chlorides may cause explosive polymerization. Contact with metals may evolve flammable hydrogen gas. Attacks some plastics, rubber and coatings May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with ammonium salts, amines forming nitrogen trichlorideНАТРИЙ дихлоризоцианурат б/в запасные части и сырье
НАТРИЙ дихлоризоцианурат б/в поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617367750398 | China | 1838 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-531-88989536 +86-15508631887 |
China | 2958 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| +8617732866630 | China | 18147 | 58 |