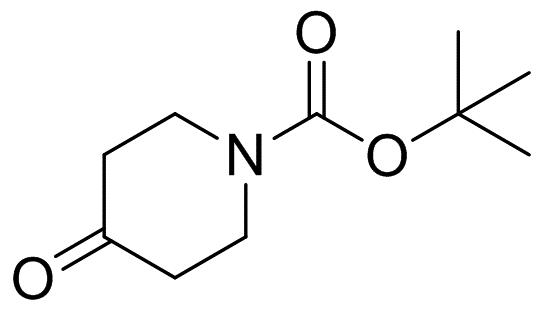
What is N-(tert-Butoxycarbonyl)-4-piperidone?
N-(tert-Butoxycarbonyl)-4-piperidone is also known as N-boc-4-piperidone, where boc is short for t-Butyloxy carbonyl. Also, boc is often adopted as a protecting group in organic synthesis.1 N-boc-4-piperidone is the derivative of 4-piperidone and is an important pharmaceutical intermediate. N-boc-4-piperidone can be used to synthesize angiotensin-converting enzyme inhibitor (ACEI) (Trandolapril), anti-AIDS protease inhibitor indinavir (Indinavir), etc. The drugs synthesized by it can also treat degenerative diseases, certain cancers and diseases caused by viral, fungi, bacterial infections, etc. 2
Fig 1. Chemical structure formula of N-(tert-Butoxycarbonyl)-4-piperidone
N-boc-4-piperidone can be synthesized by various methods, such as:
➀ 4-Piperidone hydrate hydrochloride/H2O solution was added with sodium hydroxide, di-tert-butyl dicarbonate, and THF. After 16 h of stirring at ambient temperature, the reaction mixture was extracted with diethyl ether (3 x 50 mL), and the combined organic layers were washed with brine, dried over sodium sulfate, filtered, and evaporated under reduced pressure to give N-Boc-4-piperidone as a white solid in quantitative yield (100%).3
➁ Oxidation of 1-Boc-4-hydroxypiperidine under the catalysis of (N‐heterocyclic carbene)–Ni0 system in 2,4-dichlorotoluene at room temperature for 30 min with a yield of 95%.4
N-boc-4-piperidone can be used in many reactions, and some of them are listed as follows5, 6:
➀ Synthesis of bispidines through double Mannich reaction then Wolff-Kishner-style reduction.7
➁ Synthesis of human and rat P2X7 activities for amine-substituted adamantylmethylaminebenzamides by a lithiation approach followed by addition to N-boc-4-piperidone, acid-catalyzed dehydration, and selective hydrogenation of the resulting olefin.8
➂ Synthesis of phosphates (as Scheme 1 shown) in good yield from the potassium enolates of N-boc-4-piperidone.9
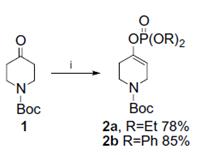
Scheme 1. Reagents and conditions: (i) 1, ClP(O)(OR)2 (1.2 equiv), HMPA (1.4 equiv), KHMDS (1.3 equiv), THF, -78 oC, 2 h.
➄ The ring expansion of N-Boc-4-piperidone (Scheme 2), which had been reported previously as having shown dangerous accumulation, exothermicity and gas evolution when conducted in diethyl ether or DCM/MTBE. This was followed by a warning that the reaction should not be attempted at a scale of more than 1 kg in batch mode for safety reasons.
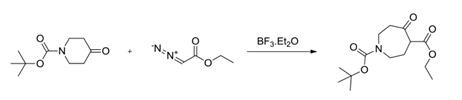
Scheme 2. Ring expansion reaction of N-Boc-4-piperidone
References
1. Ding, H. X.; Leverett, C. A.; Kyne Jr, R. E.; Liu, K. K.-C.; Fink, S. J.; Flick, A. C.; O’Donnell, C. J., Synthetic approaches to the 2013 new drugs. Bioorganic medicinal chemistry 2015, 23 (9), 1895-1922.
2. Wang, Z.; Miller, E. J.; Scalia, S. J., Modular Synthesis of Functionalized Bis-bispidine Tetraazamacrocycles. Org. Lett. 2011, 13 (24), 6540-6543.
3. Berini, C.; Winkelmann, O. H.; Otten, J.; Vicic, D. A.; Navarro, O., Rapid and Selective Catalytic Oxidation of Secondary Alcohols at Room Temperature by Using (N-Heterocyclic Carbene)–Ni0 Systems. Chemistry – A European Journal 2010, 16 (23), 6857-6860.
4. Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M., Synthesis of Extended 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives in the Suzuki Cross-coupling Reactions. Journal of Heterocyclic Chemistry 2017, 54 (2), 1550-1557.
5. Wang, R.; Zheng, Y.; Li, X.; Chen, J.; Cui, J.; Zhang, J.; Wan, X., Optically active helical vinylbiphenyl polymers with reversible thermally induced stereomutation. Polymer Chemistry 2016, 7 (18), 3134-3144.
6. http://www.chemspider.com/Chemical-Structure.643046.html?rid=f97d5cec-aa00-449e-a20c-87af1819d45c
7. https://pubchem.ncbi.nlm.nih.gov/compound/735900