Synthesis of N-Boc 4-piperidone
Reactions of N-Boc-piperidin-4-one with aromatic aldehydes afforded nitrogen-containing 1,5-diketones in the form of products of intramolecular aldol cyclization, like aza derivatives of tricyclo [7.3.1.02,7]-tridecanone system underlying naturally occurring limonoids. With o-phenylenediamine they enter into a domino-reaction leading to the formation of hydrobenzimidazo[2,1-e] acridine structure. N-Boc-piperidin-4-one in Michael reaction with diarylidenecyclohexanones forms 1,5-diketones undergoing cyclization into hydroxypyran structure.
Synthesis
We have optimized a Pd-catalyzed enantioselective borylative migration of an alkenyl nonaflate derivative of the simple precursor, N-Boc 4-piperidone. This anomalous borylation reaction lends access to a chiral optically enriched piperidinyl allylic boronate that can beemployed in carbonyl allylboration and stereoselective cross-coupling to produce substituted dehydro-piperidines related to numerous pharmaceutical agents.
A systematic fine-tuning of reaction conditions revealed that diethyl ether and the green solvent cyclopentyl methyl ether are suitable reaction solvents providing the highest enantioselectivity (up to 92% ee) under a low catalyst loading of 3 mol%. Optimization of the aldehyde allylboration step led to higher yields with further solvent economy. The multigram-scalability of the entire process was demonstrated under the reaction conditions that provide optimal atom-economy and efficiency
Current methods for the stereoselective preparation of polysubstituted piperidines can be classified into three general synthetic approaches about common synthetic strategies for the preparation of chiral piperidines:

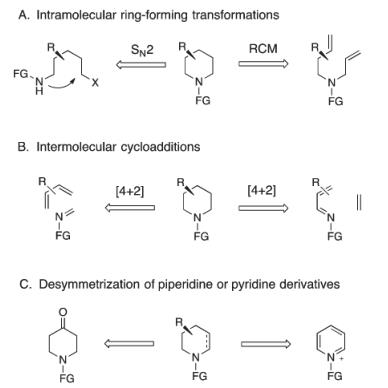
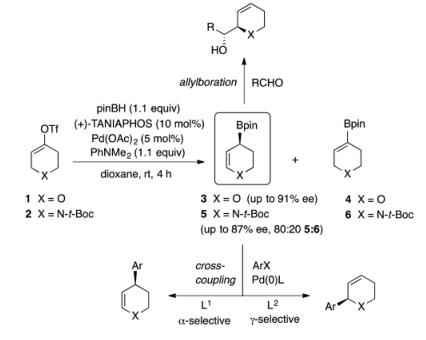
Scheme1:Synthetic applications of heterocyclic allylic boronates, prepared by a catalytic enantioselective borylative migration.
Scheme2:Optimal conditions for the racemic catalytic borylative migration of 8
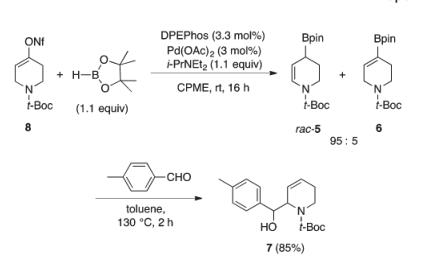
The use of alternate substrates embedding an alkenyl nonaflate or a carboxybenzyl carbamate did not lead to a significant increase of enantioselectivity; however, substrate 8 combining an alkenyl nonaflate with N-Boc protection led to a higher yield. Moreover, it is noteworthy that compared to that of substrate 2, the preparation of substrate 8 is higher-yielding and more cost-effective because perfluorobutanesulfonyl fluoride is a less expensive reagent than phenyl triflimide, and the chromatographic purification is easier. Furthermore, compared to a N-Cbz group, which is cleaved by catalytic hydrogenation, removal of a N-Boc[1] protecting group is orthogonal to the alkene in the allylboration products. Therefore, although
N-Cbz substrate 9 provided a lower proportion of undesired alkenyl boronate 11, it was decided to employ substrate 8 for subsequent optimization. The undesired alkenyl boronate side-product can be removed with ease, unreacted, after the subsequent aldehyde allylboration step.[2]
Application
The development of new, efficient and economical methods for the preparation of functionalized, optically enriched piperidines is important in the field of drug discovery where this class of heterocycles is often deemed a privileged structure.
Handling and storage
Clarify that the liquid is dark yellow. If inhaled, move the patient to fresh air., If breathing stops, perform artificial respiration. Rinse with soap and plenty of water. Wash eyes with water carefully. Never feed anything to an unconscious person., Rinse your mouth with water.
1.Inhalation if inhaled, move the patient to fresh air. If breathing stops, perform artificial respiration.
2.Skin contact wash with soap and plenty of water.
3.Eye contact wash eyes with water carefully.
4.Do not feed anything to an unconscious person. Rinse your mouth with water.
Store in a cool place. Keep the container closed and store in a dry and ventilated place. Open containers must be carefully resealed and kept upright to prevent leakage.
1. Do not allow the product to enter the sewer.
2. Place in a suitable closed container for disposal.
References
[1], "Nitrogen-Containing 1,5-Diketones Based on N-Boc-Piperidin-4-one, Synthesis, Intramolecular Cyclization, and Reaction with o-Phenylenediamine,".
[2]Kim Y. & Hall D. G., "Optimization and multigram scalability of a catalytic enantioselective borylative migration for the synthesis of functionalized chiral piperidines," Organic & Biomolecular Chemistry, Vol.14, No.20(2016), pp.4739-4748.