Olanzapine Lactam Impurity – Application
Olanzapine, chemically known as 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine is an antipsychotic drug. It is an antagonist of dopamine D-1 and D-2 receptors, in addition has antimuscarinic anticholinergic properties and antagonist activity at noradrenergic with α-receptors. It is useful in treating in psychotic conditions. Several impurities related to oxidative degradation of Olanzapine have been reported in literature and pharmacopoeia (Fig. 1). Olanzapine is amongst the most commonly prescribed antipsychotic drugs and is associated with substantial instability. 1 Olanzapine LactaM IMpurity is a degradation product of olanzapine, a serotonergic, with light yellow to yellow solid appearance (Fig. 2). Under different storage and exposure conditions, various oxidative degradation impurities of olanzapine, such as lactam and thiolactam were reported. A simple and stability-indicating reverse phase high performance liquid chromatographic method for the determination of Olanzapine and its related impurities, such as Olanzapine Lactam Impurity has been developed.2
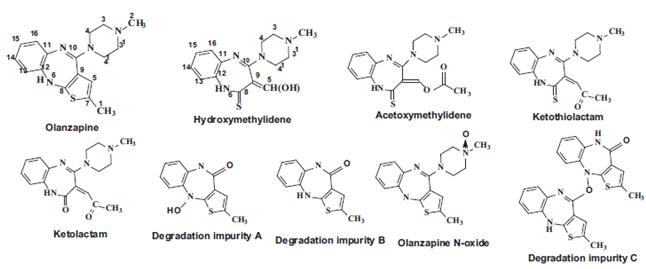
Fig 1. Olanzapine and its oxidative degradation impurities
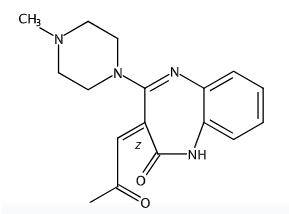
Fig 2. Chemical structure formula of Olanzapine Lactam Impurity
The synthesis of Olanzapine LactaM IMpurity from olanzapine was a multi-step shown as Scheme 1 with a yield of 7%. Further investigation suggested that the formation of Olanzapine LactaM IMpurity is a degradation product resulting from the oxidation and ring-opening of the thiophene ring of olanzapine. It’s also worth noting that the formation of Olanzapine LactaM IMpurity is typically companied by the formation of ketothiolactam. And their formation in stressed and aged solid-state formulations is proposed to occur from autoxidation processes catalyzed by formulation excipients.3
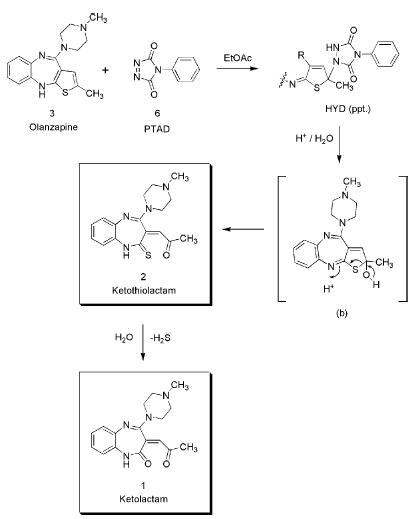
Scheme 1. Proposed pathway for preparation of Olanzapine Lactam Impurity from reaction of PTAD with olanzapine
References
1.Chue, P.; Singer, P., A review of olanzapine-associated toxicity and fatality in overdose. J Psychiatry Neurosci 2003, 28 (4), 253-261.
2.Cui, D.; Li, Y.; Lian, M.; Yang, F.; Meng, Q., Development of a simple and stability-indicating RP-HPLC method for determining olanzapine and related impurities generated in the preparative process. Analyst 2011, 136 (15), 3149-56.
3.STEVEN W. BAERTSCHI, H. B., CHARLES A. BUNNELL, GARY G. COOKE, BENJAMIN DISEROAD,DOUGLAS E. DORMAN, PATRICK J. JANSEN, CRAIG A.J. KEMP, STEVEN R. MAPLE, KAREN A. McCUNE, JEFFREY L. SPEAKMAN, Isolation, identification, and synthesis of two oxidative degradation products of olanzapine (LY170053) in solid oral formulations. J. Pharm. Sci. 2007, 97 (2), 883-892.
4. https://pubchem.ncbi.nlm.nih.gov/compound/136295210