MMBC (THIOGLO (R) 1) –Application
MMBC is the simplified form of Methyl 10-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-9-methoxy-3-oxo-3H-benzo[f]chromene-2-carboxylate. It is also commonly known as ThioGlo 1. It is a maleimide derivative of a naphthopyranone fluorophore, and would yield a fluoresecent adduct when reacted with thiols and sulfite, and the obtained product would fluresce strongly at 513 nm. ThioGlo 1 reacts with R-SH compounds quantitatively through Michael addition mechanism (1:1 on a molar basis). ThioGlo 1 emits low fluorescence prior to thiol addtion and very high fluorescene after reaction with -SH groups (ca. 50-fold increase). Also, both low molecular mass and protein-associated thiolates (R-S-) are reported to form fluorescent adducts with ThioGlo 1.1 Compared with 7-diethyla-mino-3-(40-maleimidylphenyl)-4-methylcoumarin (CPM, another fluorophore), ThioGlo 1 has several advantages, inculding a fivefold higher quantum yield of its sulfhydryl adduct (ϕ 513 nm = 0.65 versus ϕ 475nm = 0.13 for the CPM), a larger Strokes shift, and greater resistance of its maleimide moiety to hydrolysis under aqueous conditions. 2, 3
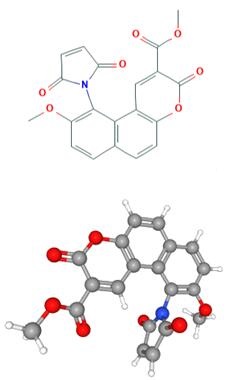
Fig 1. Chemical structure formula of MMBC
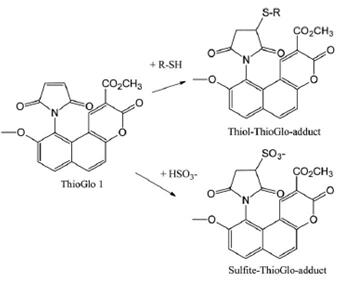
Fig 2. Reaction between ThioGlo 1 and thiol or hydrogen sulfite yielding a fluorescent adduct
ThioGlo 1 is utilized in many applications that require sulfhydryl labeling, including the quantification of the small molecular thiols, totol thiols directly on bacterial cells and natural organic matter. Some of the representative examples have been listed below:
➀ To reliably detect CoA quantities between 100 and 500 pmol in black microplates, which is comparable to the results obtained using CPM with white microplate.3
➁ To determine the content of reduced thiols in different beers using ThioGlo-1 as the thiol derivatization reagent, with the detection of the derivatized thiols by reverse-phase liquid chromatography coupled to a fluorescence detector. 4
➂ In the quantitation of cellular glutathione and protein thiol levels. And validation of the specificity of the ThioGlo 1 assay for glutathione and protein sulfhydryls was performed with cumene hydroperoxide/ glutathione peroxidase. The magnitude of the fluorescent response to glutathione was reduced by 95%, whereas the magnitude of the response evoked by sodium dodecyl sulfate-induced protein unfolding was unaffected.
However, the ThioGlo 1 labelling method is highly sensitive and selective. High levels of S2- and SO32- (>2μm) could significantly interfere with the analysis. Copper ions also affecte the determination, but this problem can be resolved by the additon of metal chelators such as EDTA. The speficity and versatility of this method are especially important as it could help in providing insights into the mechanism of complex interactions between metals and thiols under bioatic conditions. And it is recommended that the standared addtion be used to avoid complex matrix interferences and to achieve a detection limit of thiols at low micromolar levels in enviromental samples.5
References
1.Lange, R. W.; Day, B. W.; Lemus, R.; Tyurin, V. A.; Kagan, V. E.; Karol, M. H., Intracellular S-glutathionyl adducts in murine lung and human bronchoepithelial cells after exposure to diisocyanatotoluene. Chem. Res. Toxicol. 1999, 12 (10), 931-936.
2.Hoff, S.; Larsen, F. H.; Andersen, M. L.; Lund, M. N., Quantification of protein thiols using ThioGlo 1 fluorescent derivatives and HPLC separation. Analyst 2013, 138 (7), 2096-2103.
3.Collazo, E.; Couture, J.-F.; Bulfer, S.; Trievel, R. C., A coupled fluorescent assay for histone methyltransferases. Anal. Biochem. 2005, 342 (1), 86-92.
4.de Almeida, N. E.; Lund, M. N.; Andersen, M. L.; Cardoso, D. R., Beer thiol-containing compounds and redox stability: kinetic study of 1-hydroxyethyl radical scavenging ability. Journal of agricultural food chemistry 2013, 61 (39), 9444-9452.
5.https://pubchem.ncbi.nlm.nih.gov/compound/15155356
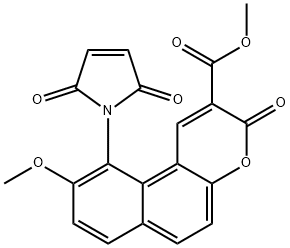 Methyl 10-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-9-methoxy-3-oxo-3H-benzo[f]chromene-2-carboxylate
Methyl 10-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-9-methoxy-3-oxo-3H-benzo[f]chromene-2-carboxylate 137350-66-4

