Dimethyl glutarate: synthesis and oxidation reaction
Introduction
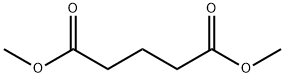
Dimethyl glutarate (Figure 1) is an environmentally friendly high boiling point solvent. This solvent has the characteristics of good solubility, low volatility, easy flow, high safety, non toxicity, and photochemical stability. It is also an important intermediate for fine chemicals. Dimethyl glutarate is used in paints, enamel, varnish, lacquer, thinner, paint stripper, remover, polyamide, polyester, resins, and plasticizers. In view of this importance of these enormous applications, its synthesis becomes essential. Electrochemical fluorination of dimethyl glutarate and its fluorine derivative has not been done so far. But, electrochemical fluorination is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds. The general approach represents an application of electrosynthesis. The fluorinated chemical compounds produced by electro-fluorination are useful because of their distinctive solvating properties and the relative inertness of carbon-fluorine bonds.[1]

Synthesis of dimethyl glutarate
There are many methods for synthesizing dimethyl glutarate, but most of them are complex and have low yields. Among them, the most commonly used method is the synthesis method using sulfuric acid as a catalyst. However, this method has disadvantages such as corrosion of equipment, multiple side reactions, complex post-treatment, and low yield, and there is an urgent need to find new catalysts.
Method 1: P-toluenesulfonic acid is a strong organic acid that has no oxidation or carbonization effects. As a catalyst for esterification reactions, p-toluenesulfonic acid has significant advantages such as high activity, non corrosive equipment, and reduced pollution.The esterification reaction between glutaric acid and methanol with P-toluenesulfonic acid as catalyst was studied.The optimum reaction conditions in 94.5% yield were as following: the molar ratio of alcohol to acid is 10:1, amount of calalyst uised 20% by weight of the glutaric acid, the reaction time is 3h under refluxing using cyclohexane as azeotropic solvent.[2]
Method 2: Dimethyl glutarate was prepared with glutaric acid and methanol as reactant, phosphotungstic acid as catalyst and toluene as azeotropic agent. The reaction mechanism was studied. Kinetic parameters were obtained by experiments carried out at various conditions. Results of experiments showed that the synthetic reaction of dimethyl glutarate appeared to be a third-order irreversible reaction. The rate constant k of the Arrhenius equation was determined. The apparent activation energy was Ea=93.133 k]/mol, and the frequency factor was k0=2.006X1010e-93133/RT(L/mol)2/min.[3]
Method 3:Dimethyl monofluoro glutarate has been successfully synthesized for the first time by electrochemical fluorination, and this method is specific toward the attachment of fluorine to the methylene group and not carbonyl carbon. Maximum yield of monofluoro product is obtained at 15 mA/cm2 . Maximum yield of 71.5% of the monofluoro product is obtained with a conversion efficiency of 95.2% when the charge of 6 F/mol is passed. The synthesized product is characterized using FTIR, GC/MS, and NMR. The product purity and composition are ascertained using GC/MS. The attachment of fluorine to methylene group is indicated using FTIR data. From NMR studies, the environment of fluorine in the neighborhood of carbon and hydrogen has been established. From the relaxation time data, it is clear that the crystal obtained is pure and homogenous.[1]
Oxidation Reaction of Liquid Dimethyl Glutarate
In this work,oxidation reaction of dimethyl glutarate exposed to radiofrequency oxygen plasma will be examined and an organic preparation method based on a new chemical concept of plasma-liquid interaction will be discussed. Dimethyl glutarate did not change without exposure to low-temperature oxygen plasma.Through a reaction period, negligible amount of reactant was evaporated and lost from the reactor vessel and trapped on liquid-nitrogen traps. After reaction, products and unconsumed reactants were analyzed by g.1.c. and gl.c.-m.s. immediately. PEG-20M terminated with 2-nitroterephthalic acid (TC-FFAP) and 100% dimethylpolysiloxane (DB-1) were used as stationary phases for g.l.c. The identification was confirmed by g.1.c.-m.s. and quantitative analysis was carried out by the internal standard method.
Possible oxygen species playing an important role to give hydroxyl-type products may be O(1D) and O(3P) atoms. O(1D) has been known to cause insertion reaction into C-H bond to form hydroxy group as well as hydrogen abstraction reaction to generate hydroxyl and alkyl radicals. On the other hand, O(3P) has been known to cause onlyhydrogen abstraction.Though its reactivity is smaller than O(1D), the contribution to the reactionshould not be neglected since O(3P) concentration in plasma may be larger than O(1D). Rich O(3P) atoms may abstract a-hydrogens from the first products in the second stage to yield hydroxyalkyl radicals.These radical intermediates may terminate with ketones as a result of hydrogen abstraction and/or disproportionation reactions. Radical coupling products were not detected.Conclusively, the reaction of dimethyl glutarate exposed to low-temparateure oxygen plasma is schematically shown in Scheme I.[4]

References
[1] Ilayaraja N , Radhakrishnan S , Renganathan N G .Electrochemical fluorination of dimethyl glutarate and its characterization[J].Ionics, 2010, 16(2):137-144.DOI:10.1007/s11581-009-0366-9.
[2] Huang MQ, et al.Study on the catalytic synthesis of dimethyl glutarate with p-toluenesulfonic acid[J].Chemical Engineer, 2006, 020(001):4-6.
[3] Sun Xb,et al. Study on kinetics of dimethyl glutarate synthesis catalyzed by phosphotungstic Acid[J]. Chemical World, 2009(5):4.DOI:10.3969/j.issn.0367-6358.2009.05.015.
[4] Yajima T, Suzuki T. Oxidation Reaction of Liquid Dimethyl Glutarate Exposed to Low-Temperature Oxygen Plasma[J].Journal of Photopolymer Science and Technology, 2005, 18(2):233-236.DOI:10.2494/photopolymer.18.233.
You may like
See also
Lastest Price from Dimethyl Glutarate manufacturers

US $0.00-0.00/kg2025-07-09
- CAS:
- 1119-40-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20 tons
US $100.00/KG2025-04-21
- CAS:
- 1119-40-0
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON