Application research of pyridine-4-boronic acid
Introduction
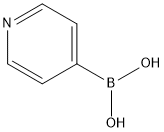
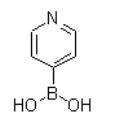
Pyridine-4-boronic acid is a kind of boronic acid derivative, of which appearance is white granular powder(Figure 1). It is useful building blocks in crystal engineering. It can also be used as a catalyst to act as a dehydrative condensation agent to synthesize amides using carboxylic acid and amines as raw materials. Its derivative, polystyrene-bound-4-pyridineboronic acid, is a useful catalyst for amidation reaction and esterification of alpha-hydrocarboxylic acids.It was already reported that 4-pyridinyl boronic acid is a zwitterionic species. In particular, the chemical shift of the pyridine protons were shifted remarkably upfield in basic medium (pH≥9), confirming that deprotonation of pyridine occurs at these pH values, and at neutral pH, pyridine-4-boronic acid exists in aqueous solution mostly in the zwitterionic form.[1]

Synthesis of Pyridine-4-boronic acid
The synthesis of pyridine-4-boronic acid by reaction of n-butyllithium with 4-Br-pyridine and successive addition of trimethoxyborane at -60℃has been previously described, but with this procedure obtained a very low yield. Furthermore, to our knowledge, the compound has not yet been fully characterized. The main problems in the synthesis arose from nucleophilic additions to 4-BrPy, which are competitive with the halogen metal exchange reaction at the relatively high temperature of -60℃,and from the low reactivity of 4-pyLi toward trimethoxyborane. To avoid these drawbacks, authors lowered the temperature to -110℃ and added TMEDA (N,N,N′,N′tetramethyl-1,2-ethylendiamine) as an activating agent. Both elemental analysis and mass spectra indicated that pyridine-4-boronic acid was isolated in the neutralform, NC5H4B(OH)2.[1]
Synthesis of [HNC5H4B(OH)2-4][PtCN4] (1)
[HNC5H4B(OH)2-4][PtCN4] (1) was synthesized by mixing of pyridine-4-boronic acid hydrochloride (34.7 mg, 0.21mmol) in 5 ml of water and K2PtCN4(0.82mg, 0.21mmol) in 10ml of water. The resulting precipitate was obtained, collected by filtration and dried. Single crystal of 1 was obtained by slow diffusion of in stoichiometric quantities of pyridine-4-boronic acid hydrochloride and [Pt(CN)4] salt reagent. 71%Yield. Elemental analysis (%). Found: C, 30.74; H, 2.58; N, 15.36. Calculated: C, 29.40; H, 2.64; N, 14.80. The structure of [HNC5H4B(OH)2-4][PtCN4] (1) compound contains the expected planar anions of [PtCN4] and pyridine-4-boronic acid cations. On the other hand, the hydrogen atoms of the boronic acids in 1 is syn-anti conformation, which is convenient for the formation of a pair of charge-assisted hydrogen bonds with the N-atoms of [PtCN4] anion.The compound (1) crystallizes in monoclinic, space group P21/c, a=5.6159(11) Å, b=14.656(3) Å, c=11.619(2) Å, α=90°, β= 110.35(3), γ=90°, V= 896.6(3) Å3, Z=2. The optimum molecular geometry parameters have been investigated with the DFT/B3LYP method. All geometric parameters are found in good agreement with crystallographic and computational results. Contributions of fragments molecular orbitals (HOFO-LUFO) to frontier molecular orbitals (HOMO-LUMO) are calculated charge transferred from Pt moiety to other fragments.[2]
Synthesis of boron-doped carbon nitride derived from pyridine-4-boronic acid
Two-dimensional g-C3N4 nanosheets have attracted tremendous attention owing to their specific surface area,high electron transport ability, significantly reduced triplet–triplet annihilation process,and potential applications.To enhance their electrochemical activity, non-metal heteroatoms,i.e., boron(B), phosphor and sulfur, were doped into g-C3N4. In particular, B and C belong to the same period and are located at an adjacent location on the periodic table, so they have many similar properties. In addition, functional groups with a B–C bond can act as Lewis acid sites. Based on this advantage, B-doped-C3N4 (BCN) has been reported by various research groups.However,these studies needed to utilize other substances (such as H3BO3 and B2O3) as a boron source and then the as-prepared precursor was calcinated to obtain the B-doped-C3N4 materials. Therefore,a convenient and simple synthesis method needs further development. The application of BCN to food quality testing has not been reported.In this work, researchers synthesized BCN nanosheets using pyridine-4-boronic acid as both a boron source and a nitrogen source, and extra impurities were not introduced through the self-doped process. This work aims to provide an innovative material to specifically detect traces of targeted analytes. There are currently three benefits to this approach: (i) BCN nanosheets are non-metallic materials with low toxicity and high stability; (ii) BCN nanosheets are successfully synthesized via one-step calcination treatment; and (iii) BCN-800 exhibits high sensitivity, selectivity, and stability in both antibody and antigen recognition.
In summary, authors suggested a facile strategy to fabricate a series of BCN composites by controlling the calcination temperature (700, 800, 900℃), which serve as an immunosensing system for detecting vomitoxin. This strategy uses pyridine-4-boronic acid as the only boron and nitrogen source. In this simple synthetic strategy, BCN-800 preserves the advantages of the non-metallic, B-doped, and unique layered structure and enhances antibody adsorption and antigen detection. Compared with the other two samples, the defect density and disorder degree of BCN-800 are between those of BCN-700 and BCN-900, which will not cause a higher defect density or higher disorder degree.In addition, the ratio of C, N, and B contents of BCN-800 is also between that of BCN-700 and BCN-800. It is because of the existence of this intermediate state that the BCN-800 material shows better electrochemical performance. Moreover, the layered stacked BCN-800 ultrathin nanosheets show excellent selectivity and sensitivity for detecting as little as 1.0 pg/mL vomitoxin. This approach may provide a new strategy for improving the synthesis of B-doped-C3N4.[3]
References
[1] Dreos R , Nardin G , Randaccio L ,et al.A Molecular Box Derived from Cobaloxime Units Held Together by 4-Pyridinylboronic Acid Residues[J].Inorganic Chemistry, 2001, 40(22):5536.DOI:10.1021/ic0102034.
[2] Elif Güngr, Sevinek R ,Hülya Kara Subasat.New Compound of Pyridine-4-Boronic Acid Cation and Pt(CN)4 Anion Salt: Synthesis, Structural Properties, Hirshfeld Surface Analysis and Density Functional Theory Calculations[J].Journal of the Institute of Science and Technology, 2021(3).DOI:10.21597/JIST.859627.
[3] Li X , Zhu P , Liu C , Pang H . One step synthesis of boron-doped carbon nitride derived from 4-pyridylboronic acid as biosensing platforms for assessment of food safety. Chem Commun (Camb). 2019;55(62):9160-9163. doi:10.1039/c9cc03787j
You may like
Related articles And Qustion
See also
Lastest Price from Pyridine-4-boronic acid manufacturers

US $10.00/kg2025-04-21
- CAS:
- 1692-15-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton

US $35.00-25.00/kg2024-01-09
- CAS:
- 1692-15-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 Ton