Detection of Ethyl p-toluenesulfonate in Drugs
Introduction
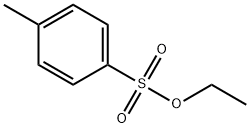
Ethyl p-toluenesulfonate is an organic chemical raw material with colorless monoclinic crystals. It is soluble in ethanol, ether, and benzene, but insoluble in water. Ethyl p-toluenesulfonate is used as an ethylation reagent in organic synthesis, in the pharmaceutical industry to produce benzyl ammonium bromide, and as an intermediate in photosensitive materials. Additionally, ethyl p-toluenesulfonate is a potential genotoxic impurity that can undergo alkylation reactions with DNA. p-Toluenesulfonic acid (pTSA), a strong organic acid, is a common reagent used in pharmaceutical industry as an "organic-soluble" acid catalyst or in purification steps of chemical synthesis of a drug substance. The applications of p-TSA include but are not limited to: Acetalization of an aldehyde, Esterification of carboxylic acids, and Transesterification of an ester. The presence of any residual alcohols like methanol and ethanol from synthetic reaction or recrystalization steps may result in the formation of corresponding alkyl tosylates, which are known to be Potential Genotoxic Impurities (PGIs). These PGIs, alkyl tosylates due to their DNA alkylation action can induce mutagenic, carcinogenic and teratogenic effects. The manufacturing process of the subject active pharmaceutical ingredient involves the solvents methanol and ethanol and p-TSA as a reagent for salt formation. Hence there is a highly likelihood for the formation of the two genotoxins, methyl-p-tolulenesulfonate, and ethyl p-toluenesulfonate (Figure 1) which cannot be ignored in the API.[1] Therefore, it is crucial to control the threshold of toxicological Concern (TTC) levels for this type of impurity.

Due to the increasing concern from the regulatory agencies with respect to potential genotoxic impurities, a number of sophisticated analytical techniques, namely Gas chromatographic methods utilizing both FID/MS detectors and HPLC/UPLC coupled with UV/MS detectors, are available for the estimation of Genotoxins in drug substances and drug products.
Rate and Mechanism in the Reactions with Water, Hydroxyl Ions and Various Halide Ions
The reactions of the alkyl halides offer a particularly striking example of this kind of exception, an example which is here extended by a quantitative study of the reactions of ethyl p-toluenesulfonate, which are closely related to the reactions of the alkyl halides. The rates of the solvolytic reactions of ethyl p-toluenesulfonate and of ethyl chloride, bromide and iodide and the rates of the displacement of toluenesulfonate ion by chloride, bromide, iodide and hydroxyl ions have been measured in 60.72% dioxane-39.28% water medium at 50°. Iodide ion displaces toluenesulfonate ion from ethyl p-toluenesulfonate at a greater rate than do the other halide ions; nevertheless, ethyl iodide, of all the ethyl halides, is most rapidly decomposed by hydrolysis. This apparent paradox may be accounted for in terms of the fact that the lower solvation energy of iodide ion approximately compensates for its weaker binding to carbon. The equilibrium constants for the displacement of toluenesulfonate ion by bromide and by iodide ion are of the order of 10. Halide ions decrease the rate of formation of ethyl alcohol from ethyl toluenesulfonate, an effect which is consistent with an ionic mechanism of solvolysis.[2]
HPLC/UV method for genotoxic impurities
A sensitive and simple HPLC/UV method has been developed and validated for the determination of two potential genotoxic impurities, namely methylp-toluenesulfonate (MPTS) and ethyl p-toluenesulfonate (EPTS) at trace levels in Pemetrexed sodium API. Applying the concept of threshold of toxicologicalconcern (TTC), a limit of 3 ppm each for both genotoxins was calculated based on the maximum daily dose of API. A reversed phase LC method using UV detection was developed and validated. The method was found to be specific and selective for the application. The limit of detection (LOD) and limit of quantitation (LOQ) for both MPTS and ethyl p-toluenesulfonate was found to be 0.15 ppm (0.009µg mL−1) and 0.5 ppm (0.03 µg/mL), respectively, with respect to sample concentration. The calibration curves of MPTS and ethyl p-toluenesulfonate were linear over the concentration range from LOQ to 6 µg/mL. The method was found to be specific, precise, linear and accurate and has been successfully applied to determine the two genotoxins in commercial batches of the API.
1.Ethyl p-toluenesulfonate in Azithromycin Granules
To establish a HPLC method for the determination of genotoxic impurity ethyl p-toluenesulfonate in azithromycin granules. The separation was performed on Waters XBridge C18 column (250mm×4.6mm, 5μm) at a flow rate of 1.0 mL/min. The mobile phase A was phosphate buffer (2.2g/L anhydrous dipotassium hydrogen phosphate solution, adjusted to pH 8.9 with dilute phosphoric acid), and mobile phase B consisted of methanol-acetonitrile (25:75) and gradient elution was performed. The detection wavelength was set at 225nm, the column temperature was 60°C , and the injection volume was 50μL. Specificity, linearity, limit of quantitation and detection, precision,repeatability, recovery, stability and durability tests are carried out. Results Blank solvent, blank excipient and impurity L nearest to ethyl p-toluenesulfonic acid in mixed solution do not interfere with ethyl p-toluenesulfonic acid detection. Good linear relationships between the concentration and the peak area of ethyl p-toluenesulfonate in the range of 0.05-0.20 μg/mL(R2=0.9999) was achieved. The limit of detection was 0.0167 μg/mL, and the limit of quantitation was 0.0500μg/mL. The average recoveries were 95.76%-96.48%, RSD were 0.42%-1.53%. Precision, repeatability, stability and durability all meet the requirements. No genotoxic impurities ethyl p-toluenesulfonate was detected in three batches of samples. This method has strong specificity, high sensitivity, high specificity and high accuracy, and can be used for the limit control and detection of ethyl p-toluenesulfonate, a genotoxic impurity in azithromycin granules.[3]
2.Ethyl p-toluenesulfonate inenalapril maleate and its intermediate
To establish a method of HPLC-QTOF MS to detect ethyl p-toluenesulfonate in enalapril maleate and intermediate.The method was achieved on a Eclipse plus C18 column( 3.0mm×100mm,1.8 μm) utilizing a mobile phase of 20 mmol/L ammonium formate( A)-acetonitrile(B) with gradient elution (0-2min,10% →50% B; 2-4min,50% →60% B; 4-7min,60% →90%B; 7-8min,90%B) at the flow rate of 1.0 mL/min.And the temperature of column was set at 50°C ; The data was acquired in positive ion mode.Standard curve was linear in the range of 0.20-5.01 μg/mL( r=0.9955).The values of limit of quantification and limit of detection were 0.4ng and 0.2ng,respectively. RSDs for the intra- and inter- day were less than 6.7%. The recoveries (n=9) of ethyl p-toluenesulfonate in enalapril maleate and intermediate were 102.6% and 90.8%,respectively.The method is convenient and sensitive for detecting ethyl p-tolu-enesulfonate in enalapril maleate and intermediate.[4]
References
[1] Nageswari A, Reddy KV, Mukkanti K. A Sensitive and Simple HPLC-UV Method for Trace Level Quantification of Ethyl p-Toluenesulfonate and Methyl p-Toluenesulfonate, Two Potential Genotoxins in Active Pharmaceutical Ingredients. Sci Pharm. 2011;79(4):865-876. doi:10.3797/scipharm.1106-23
[2] Mccleary H R , Hammett L P .Rate and Mechanism in the Reactions of Ethyl p-Toluenesulfonate with Water, Hydroxyl Ions and Various Halide Ions1[J].Journal of the American Chemical Society, 1941, 63(8).DOI:10.1021/ja01853a066.
[3]Sun X,et al.Determination of genotoxic impurity isopropyl ethyl p-toluenesulfonate inAzithromycin Granules by HPLC[J].Drug Evaluation Research,2022,45(09):1843-1847.
[4]Song DM,et al.HPLC-QTOF MS determination of ethyl p-toluenesulfonate in enalapril maleate and its intermediate[J].Chinese Journal of Pharmaceutical Analysis,2012,32(05):834-837.DOI:10.16155/j.0254-1793.2012.05.036.
You may like
See also
Lastest Price from Ethyl p-toluenesulfonate manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 80-40-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $0.00/mg2025-03-20
- CAS:
- Min. Order:
- 10mg
- Purity:
- 0.98
- Supply Ability:
- 5g