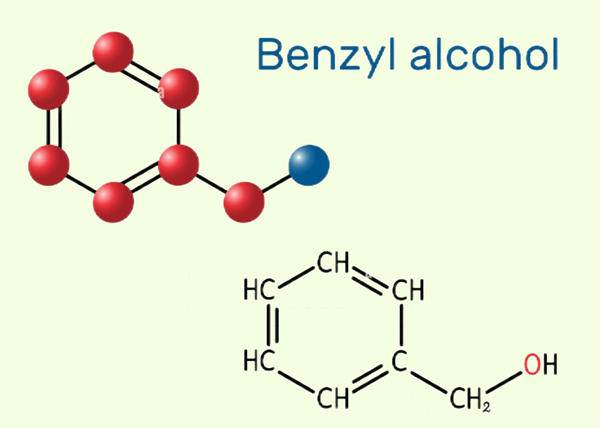
What is Benzyl alcohol?
General Description
Benzyl alcohol is also known as phenylcarbinol. Its chemical formula is C6H5CH2OH and its density is 1.045 g/mL at 25 ° C (lit). Benzylalcohol is one of the simplest fatty alcohol containing phenyl. It can be seen as benzene substituted by hydroxymethyl, or methyl alcohol substituted by phenyl. It is a colorless transparent sticky liquid with faint aroma. Sometimes benzyl alcohol is placed for a long time, it will smells like bitter almond flavor because of oxidation. Polarity, low toxicity and low steam, so it is used as alcohol solvent. It is combustible, and slightly soluble in water (about 25ml of water soluble 1 gram of benzyl alcohol). It is miscible with ethanol, ethyl ether, benzene, chloroform and other organic solvents.
Benzyl alcohol has a narcotic effect. It has a strong stimulating effect on the eye, skin and respiratory system. Ingestion, inhalation or skin contact is harmful to the body. One will feel headache, nausea, vomiting, gastrointestinal irritation, convulsion, coma after ingestion of benzyl alcohol. It can lead to death under serious condition. Median lethal dose in rats is 1230mg/kg.
When benzyl alcohol enters the body, it is firstly oxidized to benzoic acid and then condenses with glycine in the liver to form hippuric acid and excrets. Intramuscular injection using benzyl alcohol as solvent may result in gluteal muscle contracture.
Reactivity & Synthesis
Benzyl alcohol is colorless, hygroscopic, air sensitive liquid with a faint, pleasant, aromatic odor. Odor threshold concentration in water is 10 ppm (Buttery et al., 1988). Benzyl alcohol occurs in many essential oils and foods. It is a colorless liquid with a weak, slightly sweet odor. Benzyl alcohol can be oxidized to benzaldehyde, for example, with nitric acid. Dehydrogenation over a copper–magnesium oxide–pumice catalyst also leads to the aldehyde. Esterification of benzyl alcohol results in a number of important fragrance and flavor materials. Diphenylmethane is prepared by
a Friedel–Crafts reaction of benzyl alcohol and benzene with aluminum chloride or concentrated sulfuric acid. By heating benzyl alcohol in the presence of strong acids or strong bases, dibenzyl ether is formed.
Synthesis. Benzyl alcohol is manufactured mainly by two processes.
1) Benzyl chloride is hydrolyzed by boiling with aqueous solutions of alkali or alkaline-earth hydroxides or carbonates. By-product in this process is dibenzyl ether (up to 10%).
2) Toluene is, in low conversion, oxidized, with air, in the liquid phase, to benzyl hydroperoxide, which yields mainly benzyl alcohol and some benzaldehyde upon hydrolysis, for example, in the
presence of a cobalt salt. Benzyl alcohol thus obtained requires a more thorough purification for use in perfumes and flavors.
Occurance
Benzyl alcohol mainly exists in the form of free or ester in essential oil, such as jasmine oil, ylang-ylang oil, jasmine oil, hyacinth oil, sesame oil, hyacinths balsam, peru balsam and tolu balsam, which all contain this ingredient.The free alcohol is often present in several essential oils and extracts of jasmine, tobacco, tea, neroli, copaiba, Acacia farnesiana Willd., Acacia
cavenia Hook. and Arn., Robinia pseudacacia, ylang-ylang, Pandanus odoratissimus, Michelia champaca, Prunus laurocerasus, tuberose, orris, castoreum, violet leaves, clove buds and others. Also found in fresh apple, apricot, mandarin peel oil, high bush blueberry, raspberry, strawberry fruit, American cranberry and cooked asparagus.
Benzyl alcohol should not be stored for a long time. It can be slowly oxidized to benzaldehyde and anisole in the air.Therefore benzyl alcohol products often smell like almond aroma with characteristic of benzaldehyde. In addition, benzyl alcohol is also easily oxidized to benzoic acid by many kinds of antioxidants such as nitric acid.
Application
Benzyl alcohol can be used as preservative of ointments, desiccant of fibers, nylon and plastic film, stabilizer of PVC, photographic developer, solvent of acetate fiber, inks, coatings, paints, epoxy paints, dyes, casein, gelatin, shellac and the like, and intermediate to prepare the benzyl esters or ethers. It also can be used to prepare spices and flavorings (mostly benzyl alcohol ester of fatty acids), which can be used as additive of soaps, perfume, cosmetic and other products. Since the quartz and wool fibers have almostsame refractive index, it can be used to identificate them. In addition, it is used as fixative and diluent inperfume industry. The hydroxy of benzyl alcohol is very lively.

It can react with benzene to generate diphenyl methane, and with acrylonitrile to generate N-benzyl acrylamide (Ritter reaction). It also can react with phosphorus halide and halogen acid to generate benzyl halide. Benzyl halide and benzyl alcohol are benzylation (phenylmethyl) reagent. It can be used as benzyl-protecting group for carboxylic acid and alcohol hydroxy. Benzyl-protecting group can be removed by hydrogenation easily. In addition, benzyl alcohol can also be easily oxidized to benzoic acid by a variety of oxidants. If nitric acid is used, it can be oxidized to aldehyde or acid with different concentration and temperature.
Carcinogenicity
In an NTP study, F344 rats were dosed by oral gavage with 0, 200, and 400 mg/kg, 5 days/ week for 2 years. Benzyl alcohol had no effect on the survival of male rats; female rats had reduced survival, and many of the early deaths were considered related to the gavage procedure. There were no treatment-related effects on nonneoplastic or neoplastic lesions in either sex treated with benzyl alcohol. It was concluded that under the conditions of the study, there was no evidence of carcinogenic activity . In the same NTP study, B6C3F1 mice were dosed by oral gavage with 0, 100, and 200 mg/kg, 5 days/week for 2 years. No effects on survival or body weight gain were observed. There were no treatment-related effects on nonneoplastic or neoplastic lesions in either sex. It was concluded that under the conditions of the study, there was no evidence of carcinogenic activity.
Safety
Benzyl alcohol is used in a wide variety of pharmaceutical formulations. It is metabolized to benzoic acid, which is further metabolized in the liver by conjugation with glycine to form hippuric acid, which is excreted in the urine.
Ingestion or inhalation of benzyl alcohol may cause headache, vertigo, nausea, vomiting, and diarrhea. Overexposure may result in CNS depression and respiratory failure. However, the concentrations of benzyl alcohol normally employed as a preservative are not associated with such adverse effects.
Reports of adverse reactions to benzyl alcohol used as an excipient include toxicity following intravenous administration; neurotoxicity in patients administered benzyl alcohol in intrathecal preparations; hypersensitivity, although relatively rare; and a fatal toxic syndrome in premature infants.
The fatal toxic syndrome in low-birth-weight neonates, which includes symptoms of metabolic acidosis and respiratory depression, was attributed to the use of benzyl alcohol as a preservative in solutions used to flush umbilical catheters. As a result of this, the FDA has recommended that benzyl alcohol should not be used in such flushing solutions and has advised against the use of medicines containing preservatives in the newborn.
Related articles And Qustion
See also
Lastest Price from Benzyl alcohol manufacturers

US $1200.00-1100.00/ton2025-09-04
- CAS:
- 100-51-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $530.00/kg2025-08-18
- CAS:
- 100-51-6
- Min. Order:
- 1600kg
- Purity:
- 99%
- Supply Ability:
- 32 ton