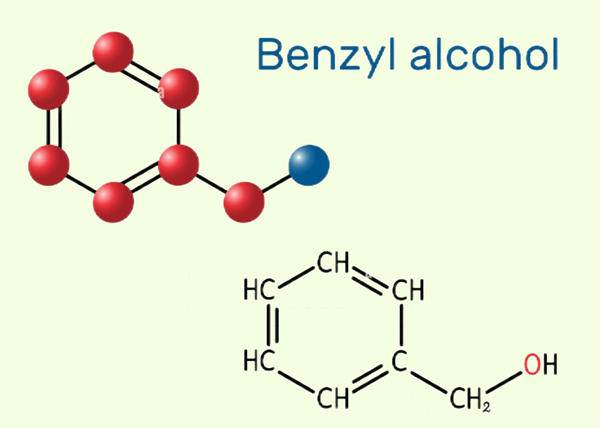
Uses of benzyl alcohol
As an important synthetic chemical that is also widely produced across nature at modest levels, benzyl alcohol was prepared chemically in small amounts from benzaldehyde and potassium hydroxide via the Cannizzaro reaction. It is now produced on industrial levels by treating benzyl chloride with sodium or potassium carbonate. The alcohol occurs naturally in a range of fruits and plants including honey, apricots, mushrooms, snap beans, cocoa, and cranberries as well as in the essential oils of plants like hyacinth and jasmine.

Uses
In the past, as much as 60% of benzyl alcohol production was used in the textile industry as a dye assistant to render hydrophobic fibers more receptive to dyes with hydrophilic properties, with direct applications to filaments like nylon, textiles, and sheet plastics. This alcohol offers among the earliest of xenobiotic biotransformation examples to be chemically characterized in the field of pharmacology. Enzymatic oxidation to benzoic acid is followed by conjugation with glycine producing hippuric acid, which was so named because the conjugate was first isolated from horse urine.
When used as a treatment for head lice, benzyl alcohol is thought to operate via a unique mechanism involving physical pulmonary obstruction of respiratory spiracles by the solvent, resulting in the asphyxiation of lice. Benzyl alcohol is a local anesthetic and produces metabolic acidosis. The latter action can be attributed to direct acidification and fixed anion effects of the metabolite benzoic acid and potentially to secondary lactic acid production due to inhibition of cellular metabolism. Weak local anesthetic effects have been related to membrane fluidization.
Mechanism of Toxicity
Although available for some years as an over the counter health product, benzyl alcohol was approved in 2003 by the Food and Drug Administration as a new prescription drug for the treatment of head lice. Unlike typical pediculicides like permethrin and lindane, which act through a neurotoxic mode of action, benzyl alcohol is thought to operate via a unique mechanism involving physical pulmonary asphyxiation. The presence of benzyl alcohol in such a wide range of consumer products is explained by its bacteriostatic and antiseptic properties in conjunction with its comparatively modest toxicity.
Environmental Fate
Due to an abundance of useful applications across society, from industrial production to consumer products, benzyl alcohol is present in the environment and is steadily released through commercial and household waste streams. Benzyl alcohol was an early object of chemists striving for greener synthetic approaches involving mixed catalysts for oxidation. It is released into the atmosphere entirely as a vapor due to its high vapor pressure, where it is lost by degradation involving reaction with hydroxyl radicals at a half-life of about 2 days. Benzyl alcohol is expected to have quite high mobility based upon its soil to water partition coefficient, and a projected soil half-life of about 13 days.
You may like
Related articles And Qustion
Lastest Price from Benzyl alcohol manufacturers

US $1200.00-1100.00/ton2025-09-04
- CAS:
- 100-51-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $530.00/kg2025-08-18
- CAS:
- 100-51-6
- Min. Order:
- 1600kg
- Purity:
- 99%
- Supply Ability:
- 32 ton