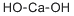Introduction of Calcium hydroxide
General description
Calcium hydroxide is a material which has been used for a variety of purposes since its introduction into dentistry in the early part of the twentieth century. In its pure form, the substance has a high pH, and its dental use relates chiefly to its ability to stimulate mineralization, and also to its antibacterial properties. A range of products has been formulated with different therapeuric actions,the effects of which are partially dependent upon the tissue to which they are applied. [1]
Application and pharmacokinetics
Calcium hydroxide has the unique potential to induce mineralization, even in tissues which have not been programmed to mineralize. It is atso likely that the calcium ions pres-ent in the applied calcium hydroxide do not become incorporated in the mineralized repair tissue, which derives its mineral content solely from the dental pulp, presumably via the blood supply. These observations indicate that calcium hydroxide is an initiator rather than a substrate for repair.
Ca(OH)2 is significantly effective in reducing endotoxin level in infected root canals. Primary root canal infection is polymicrobial in nature, pre- dominantly driven by gram-negative anaerobic bacteria. Endotoxins are the part of all gram-negative bacteria, com-posed of polysaccharides, lipids,and proteins, known as Lipopolysaccharide (LPS). [2]LPS is involved in releasing sev- eral chemical mediators of inflammation and cytokines and significantly correlates with exudate in the infected root canals, clinical signs and symptoms such as pain on palpa- tion and tenderness to percussion, and the severity of bone resorption.Calcium hydroxide is one of the most widely used intracanal medicaments; it can detoxify endotoxins by hydrolysing lipid molecules, thereby rendering it less antigenic.[3]
Calcium hydroxide-based therapeutic pads as the main material in the treatment of deep caries.Direct and indirect pulp capping, employing various materials and clinical protocols, has been used for many years to preserve the health and vitality of the pulp complex and induce pulp cells to form hard tis-sue (reparative/tertiary dentin). [4]Direct pulp capping is used when the pulp is visibly exposed (vital pulp exposure) due to caries, trauma, or iatrogenic insult such as accidental exposure during tooth preparation or caries removal. Indirect pulp capping is generally used in deep cavity preparations, with or without caries remaining, that are in close proximity to the pulp but with no visible exposure. The ultimate objectives of any pulp capping procedure should be to manage bacteria, arrest any residual caries progression, stimulate pulp cells to form new dentin, and provide a biocompatible and durable seal that protects the pulp complex from bacteria and noxious agents.[4]
Calcium ions may reduce the permeability of new capillaries, so that less intercellular serum is produced, thus increasing the concentration of calcium ions at the mineralization site. The presence of a high calcium concentration may also increase the activity of calcium-dependent pyrophosphatase, which represents an important part of the mineralization process.
Mechanism of action
The mechanism by which calcium hydrox-ide initiates the reparadve process is unclear. It has been suggested that a rise in pH as a result of the free hydroxyl ions may initiate or favour mineralization (Tronstad et al. 1981). There is a problem in accepting the hydroxyi ion as the sole initiator of the process, as it has been shown that other highly alkaline compounds such as barium hydroxide and calcium phosphate fail to initiate mineralization. However, calcium hydroxide may act as a local buffer against the acidic reactions produced by the inflammatory process . An alkaline pH may also neutralize the lactic acid secreted by Nosteociasts, and this may help to prevent further destruction of mineralized tissue.Recently, the mechanism of calcium-hydroxide-induced mineralization has been the subject of review. Ida et al. (1989) foundthat the pH of setting calcium hydroxide materials with a high pH was reduced to almost neutral when the materials were placed in contact with dentine. If this is the case, it would suggest that a high pH is unnecessary for continuous mineralized tissue formation.There does, however, appear to be some conflict between this work and that of Hassetgrenet al. (1982), who demonstrated an increase in the pH of calcium-hydroxide-treated dentine.Paradoxically, calcium hydroxide sometimes appears to stimulate resorption.Andreasen (1981) reported that external replacement resorption (ankylosis) of the roots of avulsed teeth may occur if they are replanted and filled immediately with calcium hydroxide. However, if the placement of the root filling is delayed for 2 weeks, resorption is less likely to occur.
Synthesis
Usually, calcium hydroxide prepared by the double decomposition reaction of CaCl is used as raw material. The purity is as high as 99.9%, the diameter distribution is uniform, and the particle size distribution is uniform. There are few impurities that affect the reaction process, but the cost is high. According to the stoichiometric ratio, About 0.67g Ca (OH) 2 can be prepared by re decomposition of 1G CaCl, so it is suitable, so it is not suitable for the amount of Ca (OH) 2. A widely used process. Calcium hydroxide (Ca (OH) 2) in the calcium oxide industry is often made of stone, usually with quicklime (CAO) as raw material, and the water should be digested with water. Its cost and material are suitable. A large number of spare smoke places, building and reconstruction are low in oxygen and easy to obtain raw materials. It is suitable for mass preparation and use, usually flue gas treatment, building repair All hydroxides used in paper-making fillers are made of ash. Calcium is made of limestone. After calcination, crushing and impurity removal, quicklime (CAO) is obtained. It is digested with water and then reacts with the chemical system. Its stoichiometric relationship is as follows. Due to the need to add more heat of water on the basis of theory, part of the water is vaporized and water consumption. Therefore, it is usually necessary to add an appropriate amount of water on the basis of theoretical value to complete the reaction. According to the differences of production process and reaction system, Cao hydration and water digestion into Ca (OH)2 work are divided into dry digestion (dryhydration and legalization) and wet digestion.[5]
References
1 A review of calcium hydroxide November 1990 Pages 283-297
2 Sadaf D, Ahmad MZ. Calcium hydroxide (CA[OH]2) as an intracanal medication may significantly reduce endotoxins level from level from infected teeth. J Evid Based Dent Pract. 2021 Sep;21(3):101616.
3 Chavez de Paz LE. Redefining the persistent infection in root canals: possible role of biofilm communities. J Endod. 2007 Jun;33(6):652-62.
4 Tamara Georgievna Tsagaraeva,Madina Kazbekovna Slanova,Tamiris Aslanovna Aldatova,Amina Ahmedova Kurueva,Evgeniya Nikolaevna Semkina,Edita Garnikovna Margaryan,Kristina Timurovna Tamoeva,Konstantin Anatolevich Zenin,Sergey Nikolaevich Povetkin. Calcium Hydroxide-based Therapeutic Pads as the Main Material in the Treatment of Deep Caries[J]. Journal of Pharmaceutical Research International,2021
5 Yan Wenli. Study on preparation process and carbonization product properties of calcium hydroxide [D]. East China University of science and technology, 2021
You may like
Related articles And Qustion
Lastest Price from Calcium hydroxide manufacturers

US $0.00-0.00/kg2025-07-07
- CAS:
- 1305-62-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 1305-62-0
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt