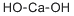Which base is more stronger NaOH or Ca(OH)2 ?
Calcium hydroxide is more basic when the concentrations are the same. This is due to the fact that the pH depends on the concentration, so assuming we have two solutions of Ca(OH)2 and NaOH at the same concentration, Calcium Hydroxide has 2 OH-'s available to donate, whereas Sodium Hydroxide has only 1 OH-'s, therefore Calcium Hydroxide is more basic. However, sodium hydroxide is usually considered stronger than calcium hydroxide if concentration is not taken into account. This is because sodium hydroxide dissociates completely in water, while calcium hydroxide only partially dissociates. Calcium hydroxide, though, is less soluble in water. However, it is still 100% ionised into calcium and hydroxide ions. Therefore, calcium hydroxide is still considered a strong base.
You may like
Related articles And Qustion
See also
Lastest Price from Calcium hydroxide manufacturers

US $0.00-0.00/kg2025-07-07
- CAS:
- 1305-62-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 1305-62-0
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt