Benzyl alcohol: Application, metabolism and toxicity
General description
Sodium benzoate is the sodium salt of benzoic acid and exists in this form when dissolved in water. It can be produced by reacting sodium hydroxide with benzoic acid, and it is a widely used food pickling agent. Sodium benzoate can be found in many natural food sources. Fruits and vegetables can be rich sources, particularly berries such as cranberry and bilberry. Other sources include seafood, such as prawns, and dairy products like milk, cheese, and yogurt. Its appearance is as follows:

Figure 1 Appearance of Sodium benzoate.
Application
Sodium benzoate was the first preservative allowed by the FDA for use in food products. Antimicrobial activity of benzoate is related to pH, so that most of its activity occurs at low pH. Sodium benzoate is used mainly as a preservative in margarine, salad dressings, marinades, cider, soft drinks, pickles, fruit salad, wafers, bakery products, jams, jellies, juices, biscuits, cakes and muffins, tomato paste and soy sauce [1]. Sodium benzoate has been reported to be used in various cheeses and in some caviar. There are also reports on the use this substance in wine and beer and olives [2]. Sodium benzoate is detectable in foods in the laboratory by a spectrophotometer at a wavelength of 224 nm [2]. Sodium benzoate is used in the pharmaceutical industry in syrups, in containers used for preparation of liquids, to prepare tablets, and at 0.2 to 0.4% to make tablets transparent and smooth and to allow rapid decomposition of the tablets. The maximum concentration of sodium benzoate used in the pharmaceutical industry is 5% and is also used in cosmetics products.
Pharmacokinetics
Sodium benzoate in the mitochondria of liver cells is metabolized by binding to the amino acid glycine and excreted as hippuric acid from the urine. Natural excretion of hippuric acid in urine is approximately 1-2 mL and 400-800 mg of glycine is excreted as hippuric acid in the urine, daily [3]. In a study conducted during year 2012 on 3083 Belgian consumers, it was concluded that these people use 1.25 mg/kg of sodium benzoate daily and this amount is 0.25% of the acceptable daily limit and is enough to bind with 50 mg of glycine and excreted as hippuric acid [3]. After entrance of sodium benzoate to the body, the intestinal cycle occurs, and sodium benzoate binds to trypsin and can increase the activity and result in the restructure of trypsin. Trypsin is released from the pancreas and has an important role in the digestion of food products. However, this has not been fully proven and requires further studies and research [4].
Toxicity
Although sodium benzoate is accepted as a safe substance, but short-term exposure can cause irritation of eyes, skin and respiratory tract, yet prolonged or repeated contact may cause high skin sensitization [5]. Using high doses causes release of histamine and prostaglandin, ulcers and gastric mucus secretion changes [6]. In a study conducted during year 2007, sodium benzoate increased blood pressure, eventually tearing the vessels in the blood cells of the rat [7]. Damage to the hepatocyte cell membrane and crista losses in mitochondria, connection to outer shell of vacuole mitochondria in the cytoplasm and liver and kidney dysfunction are other adverse effects of consuming sodium benzoate. Research has also shown the formation of benzene in the reaction between benzoic acid and ascorbic acid in the presence of metal catalysts in soft drinks and fruit juices.
Another study showed that sodium benzoate at concentration of 200 mg/kg can decrease weight in mice and increase creatinine, urea and uric acid in the isolated serum from mice [8]. Fujitani et al. in a study on rats and mice showed that at a concentration of 2.4%, the average weight of the rats was reduced compared to the control group; weight gain in kidney and liver occurred at a concentration of 2.4% in rats and liver and kidney weight increased when compared with the control group at 3% concentration in the mice [9].
References
[1]Hong H, Liang X, Liu D. Assessment of benzoic acid levels in milk in china. Food Control. 2009;20(4):414–8.
[2]El-Ziney MG. GC-MS analysis of benzoate and sorbate in saudi dairy and food products with estimation of daily exposure. J Food Technol. 2009;7(4):127–34.
[3]Beyoglu D, Idle JR. The glycine deportation system and its pharmacological consequences. Pharmacol Ther. 2012;135(2):151–67.
[4]Mu Y, Lin J, Liu R. Interaction of sodium benzoate with trypsin by spectroscopic techniques. Spectrochim Acta A Mol Biomol Spectrosc. 2011;83(1):130–5.
[5]Nascimento Filho I, Schossler P, Santos Freitas L, Melecchi MI, Rodrigues Vale MG, Bastos Caramao E. Selective extraction of benzoic acid from land?ll leachate by solid-phase extraction and ionexchange chromatography. J Chromatogr A. 2004;1027(1-2):167–70.
[6]Kreindler JJ, Slutsky J, Haddad ZH. The e?ect of food colors and sodium benzoate on rat peritoneal mast cells. Ann Allergy. 1980;44(2):76–81.
[7]Eberechukwu S, Amadikwa A, Okechukwu M. E?ect of oral intake of sodium benzoate on some haematological parameters of wistar albino rats. Sci Res Essays. 2007;2(1):6–9.
[8]Lu N, Shen M. Research on mutagenicity of sodium benzoate in born marrow cells. J Jilin Agri Univ. 2006;28(4):466–8.
[9]Fujitani T. Short-term e?ect of sodium benzoate in F344 rats and B6C3F1 mice. Toxicol Lett. 1993;69(2):171–9.
General description
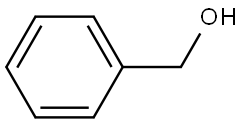
Benzyl alcohol is an aromatic alcohol. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide. Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Its appearance is as follows:

Figure 1 Appearance of Benzyl alcohol.
Application
Benzyl alcohol is a fragrance ingredient used in many compounds. It may be found in fragrances used in decorative cosmetics, fine fragrances, shampoos, toilet soaps and other toiletries as well as in non-cosmetic products such as household cleaners and detergents. It is a colorless liquid with a faint, nondescript odor, rather sweet, but varies considerably with the quality of the alcohol [1]. This material has been reported to occur in nature, with highest quantities observed in species of allium plants. The worldwide volume of use for benzyl alcohol is in the region of 100–1000 metric tons per year. This reported volume is for its use as a fragrance ingredient in fragrance compounds (mixtures) found in all finished consumer product categories. The volume of use is surveyed by IFRA approximately every 4 years through a comprehensive survey of IFRA and RIFM member companies. As such the volume of use data from this survey provide volume of use of fragrance ingredients for the majority of the fragrance industry. Moreover, benzyl alcohol is
Metabolism
When benzyl alcohol was injected i.p. into three female adult rats at 0.41 mM per animal, hippuric acid excretion in urine accounted for 41.7%, 53.7%, and 78% (aver- age: 57.8) of the administered dose. Samples were analyzed by thin layer chromatography [3]. At a dosing level of 0.25 g/kg, the urinary metabolites of benzyl alcohol consisted of 2% (0–4) mercapturic acid, 74% (71– 76%) glycine conjugate, 8% (4–15%) copper reducing material (as glucuronic acid), 14% (6–23%) glucosiduronic acid, and no ethereal sulfate. Ether soluble acid accounted for 84% (80–90%) of the administered dose. Mercapturic acid plus the glycine conjugate accounted for 76%. Benzyl alcohol was not present at measurable levels in the blood of treated animals, but its oxidation product benzoic acid was present in significant amounts. Systemic exposure (as indi- cated by AUC values) was broadly proportional to the administered doses, and maximum plasma levels were achieved 0.5–2 h after administration. Results were similar in male and female rats.
Toxicity
In a study of the acute oral toxicity of 107 flavor ingredients, it was reported that the LD50 for benzyl alcohol in 10 Osborne-Mendel rats was 1.23 g/kg (1.13–1.33 g/kg) in fasted rats and 1.58 g/kg (1.41–1.77 g/kg) in unfasted mice. Test article was administered undiluted to rats and at 25% in corn oil to mice. Clinical signs of toxicity in rats included coma and depression within 10–15 min with death times occurring between 1 h and 4 days. Clinical signs of toxicity in mice included depression, with animals dyeing within 18 h [4].
References
[1]Bar, V.F., Griepentrog, F., 1967. Die Situation in der gesundheitlichen Beurteilung der Aromatisierungsmittel fur Lebensmittel. Medizin Ernahr 8, 244–251.
[2]Michael Ash; Irene Ash. 2004. Handbook of Preservatives. Synapse Info Resources. p. 292.
[3]Teuchy, H., Quatacker, J., Wolf, G., VanSumere, C.F., 1971. Quantitative investigation of the hippuric acid formation in the rat after administration of some possible aromatic and hydroaromatic precursors. Archives Internationales de Physiologie et de Biochimie 79, 573–587.
[4]Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L., Fitzhugh, O.G., 1964. Food flavorings and compounds of related structure I. Acute oral toxicity. Food and Cosmetics Toxicology 2 (3), 327–343.
You may like
Related articles And Qustion
See also
Lastest Price from Benzyl alcohol manufacturers

US $1200.00-1100.00/ton2025-09-04
- CAS:
- 100-51-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $530.00/kg2025-08-18
- CAS:
- 100-51-6
- Min. Order:
- 1600kg
- Purity:
- 99%
- Supply Ability:
- 32 ton