tert-Butyl methyl ether: Application, synthesis and hazard
General description
tert-Butyl methyl ether (MTBE) is a volatile, flammable, and colorless liquid that is sparingly soluble in water. It is used as a fuel component in fuel for gasoline engines. It is one of a group of chemicals commonly known as oxygenates because they raise the oxygen content of gasoline. Primarily used as a fuel additive, tert-Butyl methyl ether is blended into gasoline to increase knock resistance and reduce unwanted emissions. tert-Butyl methyl ether production in the U.S. peaked in 1999 at 260,000 barrels per day before dropping down to about 50,000 barrels per day and holding steady, mostly for the export market. Worldwide production capacity of tert-Butyl methyl ether in 2018 was estimated to be 35 million metric tons. Its appearance is as follows:

Figure 1 Appearance of tert-Butyl methyl ether.
Application
tert-Butyl methyl ether has many uses in the industrial applications. It is extensively used in industry as a safer alternative to diethyl ether (which is commonly used in academic research) as the tert-butyl group prevents tert-Butyl methyl ether from forming potentially explosive peroxides. It also is used as a solvent in academic research, although it is used less commonly than diethyl ether [1]. Although an ether, tert-Butyl methyl ether is a poor Lewis base and does not support formation of Grignard reagents. It is also unstable toward strong acids. It reacts dangerously with bromine [2]. Moreover, tert-Butyl methyl ether is used as anti-knocking agent. In the U.S. tert-Butyl methyl ether has been used in gasoline at low levels since 1979, replacing tetraethyllead (TEL) as an antiknock (octane rating) additive to prevent engine knocking. Oxygenates also help gasoline burn more completely, reducing tailpipe emissions and dilute or displace gasoline components such as aromatics (e.g., benzene). Before the introduction of other oxygenates and octane enhancers, refiners chose tert-Butyl methyl ether for its blending characteristics and low cost.
Synthesis
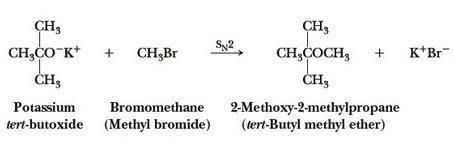
tert-Butyl methyl ether can be synthesized according to the previous work [6]. In a typical experiment, the reactor was first charged with 25 cc of 12-molybdophosphoric acid-on-titania extrudates, prepared as described supra. A screen of glass beads was placed at the top and bottom of the reactor to ensure the that extrudates would remain in the middle portion. The catalyst bed was first conditioned overnight by washing with methanol/tert-butanol (2:1 mix) at 100VC, 20 bar back pressure and a liquid flow rate of 25 cc/h. The same solution of methanol (1281.6 g, 40.0 mole) plus tert-butanol (1482.4 g, 20.0 mole) was then pumped through the catalyst bed at 25 cc/h, while the reactor was held at 100VC, at a total pressure of 20 bar. tert-Butyl methyl ether was taken periodically, either by trapping in a dry ice cooled container, or by collecting on-stream (on line) in a 316 ss bomb. Yeild: 86%.
Hazard in environment
tert-Butyl methyl ether gives water an unpleasant taste at very low concentrations. It often is introduced into water-supply aquifers by leaking underground storage tanks (USTs) at gasoline stations or by gasoline containing tert-Butyl methyl ether being spilled onto the ground. The higher water solubility and persistence of tert-Butyl methyl ether cause it to travel faster and farther than many other components of gasoline when released into an aquifer [3]. tert-Butyl methyl ether may be removed rapidly and economically from water to undetectable levels. Activated carbon produced from coconut shells and optimized for tert-Butyl methyl ether adsorption may reduce it to undetectable levels, although this level of reduction is likely to occur only in the most ideal circumstances.
There are currently no known published cases of any in-situ treatment method that has been capable of reducing contaminant concentrations to baseline (pre-development) conditions within the aquifer soil matrix. According to the IARC, a cancer research agency of the World Health Organization, tert-Butyl methyl ether is not classified as a human carcinogen. It may be tasted in water at concentrations of 5 – 15 µg/l [4]. As of 2007, researchers have limited data about the health effects of ingestion of tert-Butyl methyl ether. The United States Environmental Protection Agency (EPA) has concluded that available data are inadequate to quantify health risks of tert-Butyl methyl ether at low exposure levels in drinking water, but the data support the conclusion that it is a potential human carcinogen at high doses [5].
References
[1]Matyash, V.; Liebisch, G.; Kurzchalia, T. V.; Shevchenko, A.; Schwudke, D. (2008). Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. The Journal of Lipid Research. 49 (5): 1137–1146.
[2]Interaction between bromine and tert-butyl methyl ether. UK Chemical Reaction Hazards Forum. Archived from the original on 13 March 2011. Retrieved 13 May 2010.
[3]San Francisco Bay Area Regional Water Quality Control Board Integrated Basin Management Plan (2004) Archived 2008-02-29 at the Wayback Machine
[4]Fischer A, Oehm C, Selle M, Werner P (2005). Biotic and abiotic transformations of methyl tertiary butyl ether (MTBE). Environ Sci Pollut Res Int. 12 (6): 381–6.
[5]California Reformulated Gasoline Phase 3. Sacramento, CA: California Air Resources Board. 2015-07-24.
[6]Knifton (1999). Methyl tert-butyl ether synthesis from tert-butanol via inorganic solid acid catalysis. Edwards / Applied Catalysis A: General 183: 1-13.
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butyl methyl ether manufacturers

US $0.00/kg2025-05-27
- CAS:
- 1634-04-4
- Min. Order:
- 230kg
- Purity:
- ≥99%
- Supply Ability:
- 200000

US $10.00/KG2025-04-21
- CAS:
- 1634-04-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt