Urea: Application, toxicity, storage, Preparation
Indication
Urea is a colorless, odorless solid that contains 46% nitrogen and has the formula CO(NH2)2. It is a key compound in the metabolism of nitrogen-containing compounds in mammals and is produced naturally in the liver as a waste product of protein metabolism. Urea is widely used in the fertilizer industry as a source of nitrogen for plants, as well as in the production of plastic resins, animal feed, and as a raw material in various chemical processes. It is also commonly used in the manufacturing of skincare and haircare products due to its hydrating and exfoliating properties[1]. As a major nitrogen fertilizer, urea is an important chemical fertilizer for grain growth. Its supply is directly related to the food security of a country and region, and it has become a strategic material. In 2020 alone, China's urea export volume was 5.45 million tons. With more than 50 years of production experience in urea production in China, through introduction, digestion, and innovation, all urea production processes in the world have been basically assembled, and rich practical production experience has been accumulated. Through research, development, and innovation, various production processes unique to China have been formed; With the gradual maturity of the process, the consumption of raw materials has approached the theoretical level. However, due to the energy crisis and increasingly fierce industry competition, reducing system energy consumption, increasing system capacity, and operating cycle has become a trend in urea production today.

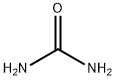
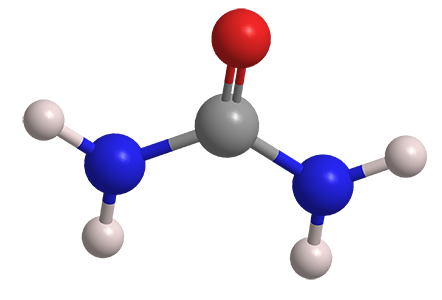
Figure 1 Appearance of urea
Application
Urea is widely used in agriculture, livestock industry, industry, medicine, environmental protection, and other fields. Urea is widely used as a nitrogen-rich fertilizer for plants, especially in agriculture. It provides a readily available source of nitrogen which helps in the growth and development of plants. Urea is a high concentration organic nitrogen fertilizer. After urea is applied to the soil, it is hydrolyzed into NH3 and carbon dioxide under the action of water and microorganisms. NH3 is nitrated into nitrate under the action of bacteria, which is absorbed by the crops. Carbon dioxide is also absorbed by the crops, promoting photosynthesis, leaving no residue in the soil, and does not acidify the soil. Urea is also used as a feed supplement for ruminant animals like cattle, sheep, and goats. It is an important source of non-protein nitrogen (NPN) and is added to animal feed to improve its protein content[2]. urea is used as an additive in the production of a variety of chemical products, mainly as a synthetic polymer material. More than half of them produce urea formaldehyde resin and melamine, which are used in the production of plastics, paints, adhesives, etc. In addition, other important organic chemical materials can also be synthesized through urea, such as dimethyl carbonate, urea peroxide, etc. Urea is used as a key ingredient in diesel exhaust fluid (DEF). DEF is used in diesel engines to reduce emissions of nitrogen oxides (NOx) which are harmful pollutants. Urea exists in the cuticle of the skin and is the main component of the skin's natural moisturizing factor. It can be used as an additive for moisturizing ingredients such as facial mask, skin lotion and hand cream in cosmetics. As a stable and non-toxic solid material, urea has high denitrification efficiency and is an ideal source of ammonia for denitrification[3]. Therefore, it is widely used in selective catalytic reduction (SCR) denitrification.
Toxicity
Urea is generally considered to be of low toxicity to humans and animals. However, exposure to large amounts of urea can cause toxicity and potentially harmful effects. Ingestion of urea may result in gastrointestinal irritation, vomiting, and nausea. Skin contact with urea can cause irritation, redness, and itching. Inhalation of high concentrations of urea dust or vapor can lead to respiratory problems such as coughing and shortness of breath.
In addition, long-term exposure to urea may have adverse effects on the environment. When urea is applied to soil as fertilizer, it can contribute to the pollution of groundwater by leaching into waterways. Urea can also promote the growth of algae in bodies of water, which can cause oxygen depletion and harm aquatic life.
Overall, while urea is generally considered safe when used appropriately, caution should be taken to avoid excessive exposure to urea and to properly dispose of any unused product.
Storage
Urea should be kept in a cool, dry place away from sources of heat and flame. It is important to ensure that the container used for storage is properly sealed to prevent moisture from entering, as this can cause the compound to break down and lose its effectiveness over time. Proper handling and storage of urea are crucial to ensuring its safety and efficacy in various applications.
Preparation
The synthesis of urea involves the reaction between ammonia and carbon dioxide. The chemical equation for the reaction is shown in the second figure. This reaction takes place at high temperature and pressure in the presence of a catalyst such as hydrated iron(III) oxide. The process can be performed in two main steps: Carbon dioxide and ammonia are compressed and pumped into an autoclave, which is a large pressurized vessel. The mixture is heated to around 180-200 ℃ under a pressure of 150-200 atmospheres in the presence of a catalyst. As the reaction proceeds, water vapor is removed from the mixture to drive the equilibrium further towards the production of urea. After completion of the reaction, the urea is separated, purified, and dried to produce a solid white powder.
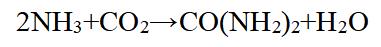
Figure 2 Chemical reaction equation of urea
References
[1] Brouwer E, Stegeman H, Kema I P, et al. Concentrations of urea in blood, milk, and urine of dairy cows as influenced by protein intake and lactational stage. Journal of dairy science, 96(10), 6355-6366.
[2] Zhang Min, Diao Qiyu. Study on the application of non protein nitrogen urea in ruminants [J]. China Feed, 2002 (05): 6-8.
[3] Wang Jiaming. Overview of New Fields of Urea Application and Its Development Prospects [J]. Chemical Industry, 2013,31 (11): 24-28.
[4] Li Shan shan. Comparison of urea synthesis processes [J]. Science and Technology Information Development and Economics, 2010,20 (11): 215-217.
You may like
Related articles And Qustion
Lastest Price from Urea manufacturers
US $0.00-0.00/kg2025-12-08
- CAS:
- 57-13-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $280.00-260.00/ton2025-07-15
- CAS:
- 57-13-6
- Min. Order:
- 25ton
- Purity:
- N46
- Supply Ability:
- 5000tons