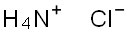Ammonium chloride: Pharmacology, Applications, Toxicity, storage, stability, preparation
Introduction
Ammonium chloride, also known as sal ammoniac, is an inorganic salt with the chemical formula NH4Cl. It is a white crystalline solid that is highly soluble in water. Ammonium chloride is a mildly acidic salt that reacts with strong bases to form ammonia gas. It sublimes upon heating, meaning it transitions directly from a solid to a gas without melting. It decomposes into ammonia and hydrogen chloride gases when heated with alkali metal hydroxides or carbonates. Ammonium chloride is not considered toxic at low concentrations. However, inhalation of high concentrations of ammonium chloride dust can cause irritation to the respiratory tract. Ingestion of large quantities of ammonium chloride may lead to nausea, vomiting, and gastrointestinal irritation. Ammonium chloride has various applications in different fields[1].

Figure 1 chemical formula of Ammonium chloride
Pharmacology
Pharmacology of Ammonium Chloride:
Ammonium chloride is an inorganic compound commonly used to treat respiratory and urinary tract disorders. Its pharmacology mainly includes the following aspects: Ammonium chloride can produce acidic substances through metabolism, thereby lowering the pH value of urine. This effect helps prevent stone formation and also promotes the excretion of certain drugs. Ammonium chloride can stimulate the respiratory center and increase the secretion of respiratory tract mucus, helping to relieve symptoms of respiratory tract diseases. Ammonium chloride can promote liver cell metabolism, enhance liver function, and effectively combat liver failure and cirrhosis. Ammonium chloride has the ability to inhibit bacterial growth and can be used to treat bacterial infections such as urinary tract infections.
It should be noted that although Ammonium Chloride is a commonly used drug, appropriate dosage and duration of use should be determined by a doctor to avoid adverse reactions[2].
Pharmacokinetics of Ammonium Chloride:
Ammonium chloride is a salt that is soluble in water and is readily absorbed by the body. When ammonium chloride is ingested, it dissociates into its constituent ions, ammonium (NH4+) and chloride (Cl-), which are absorbed in the gastrointestinal tract.
The absorption of ammonium chloride occurs mainly in the small intestine, with only a small amount being absorbed in the stomach. The rate of absorption is rapid, with peak levels of ammonium in the blood occurring within one hour after ingestion. Once absorbed, ammonium is metabolized in the liver to urea, which is then excreted by the kidneys in the urine. The chloride ion is also excreted in the urine or may be reabsorbed in the kidneys depending on the body's needs.
The elimination half-life of ammonium chloride is about 2-3 hours, indicating that it is rapidly eliminated from the body. The pharmacokinetics of ammonium chloride can be affected by factors such as age, renal function, and other medications that may interfere with its metabolism or excretion. Overall, ammonium chloride is well-absorbed and quickly metabolized and eliminated from the body. It should be noted that the pharmacokinetic characteristics of Ammonium Chloride may be affected by factors such as patient age, health status, and dosage, so adjustments should be made according to specific circumstances when using it[3].
Applications
Ammonium chloride is a source of nitrogen for fertilizers and is particularly suitable for crops that require high levels of nitrogen, such as wheat, barley, and rice. It is also used in hydroponic systems to provide essential nutrients to plants.
It helps conduct electricity between the electrodes as an electrolyte. It is particularly useful in zinc-carbon batteries where it helps to prevent the formation of hydrogen gas during the battery's discharge cycle. In the textile industry, it is used as a dyeing assistant to help fix dyes onto fabrics. It can stabilize pigments and improve their adhesion to fabrics as a printing assistant.
Ammonium has a wide range of applications in various industries. One of its primary uses is as a fertilizer, as it provides essential nutrients such as nitrogen to plant growth. In addition to agriculture, ammonium is also used in the production of various chemicals, such as explosives and pharmaceuticals. It can be used as a flame retardant in plastics and textiles, as well as a refrigerant in air conditioning systems. Ammonium compounds are also used in water treatment processes to remove impurities and contaminants. Overall, ammonium plays a vital role in many industries and is an essential component in the production of numerous everyday items.
Toxicity
The toxicity of ammonium chloride can vary depending on the dosage and route of exposure. Ingestion of large amounts of ammonium chloride can cause nausea, vomiting, abdominal pain, and even respiratory failure. Exposure to high concentrations of ammonium chloride vapor or dust can also cause irritation to the eyes, skin, and respiratory system[7].
Storage
Ammonium chloride is a chemical compound that has a strong tendency to absorb moisture. Therefore, when storing it, one should take care to avoid damp conditions. Furthermore, exposure to sunlight can cause it to decompose and release toxic gases, which makes it important to keep it away from direct sunlight, as well as heat sources and high temperatures. Additionally, mixing ammonium chloride with certain substances can result in hazardous reactions, including the production of harmful gases or explosive compounds, so it is imperative to store it separately from other chemicals. Finally, it is crucial to ensure that the packaging remains intact, without any leaks or punctures, in order to prevent accidents. In summary, proper storage of ammonium chloride requires keeping it in a dry and well-ventilated area, protecting it from moisture, light, heat, fire, and incompatible materials.
Stability
Ammonium chloride is generally stable at room temperature, but it can decompose under high temperatures or in damp conditions. Heating ammonium chloride beyond its decomposition point results in a decomposition reaction that releases ammonia and HCl gases, which can be harmful to humans. In addition to high temperatures, ammonium chloride can react with certain substances such as alkaline materials, oxidizing agents, and copper, producing hazardous gases or explosive compounds.
Preparation
To produce ammonium chloride, ammonia gas can be passed through concentrated hydrochloric acid to create a white solid precipitate. The mixture is heated to the appropriate temperature, and the hydrochloric acid solution is kept cold in an ice bath. When ammonia gas is introduced into the flask, it reacts with the hydrochloric acid to form ammonium chloride, which will appear as white crystals at the bottom of the flask. After the ammonium chloride has formed, it can be rinsed with a small amount of hydrochloric acid to remove impurities and then with pure water. The final product can be obtained by drying the ammonium chloride crystals. It's important to handle ammonia gas and hydrochloric acid with care to avoid any potential hazards.
References
[1] El-Azzami M, et al. Preparation of Ammonium Chloride from Industrial Waste Gas Streams Containing Ammonia and Hydrochloric Acid. Journal of Cleaner Production, 2018, 189: 465-473.
[2]Yamada T, et al. Effectiveness of ammonium chloride in preventing recurrence after the first calcium phosphate stone.[J] Urology, 74(3): 509-513.
[3] Rennick GJ, et al. Absorption and metabolism of ammonium chloride in humans.[J] Clinical Science, 77(6): 651-658.
[4] Rashid A, Mahmood T, Anwar M, et al. Ammonium Chloride Fertilizer: Its Application and Usage in Sustainable Agriculture. [J] Journal of Chemical Society of Pakistan, 39(5), 636-641.
[5] Ashrafuzzaman M, Islam, M T. Application of Ammonium Chloride in Textiles. [J] Bangladesh Journal of Scientific and Industrial Research, 51(3), 211-220.
[6] Sahoo P K, Kim J H. Ammonium Chloride as a Suitable Flux for Refining of Zinc from Galvanized Iron Scrap.[J] International Journal of Minerals, Metallurgy, and Materials, 23(11), 1278-1284.
[7] European Food Safety Authority (EFSA) (2005). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to Ammonium Chloride (E510). EFSA Journal, 223, 1-25.
[8] Dodd J H, Lyons L E. The Preparation of Ammonium Chloride. Journal of Chemical Education, 1950, 27(5): 239-240.
Related articles And Qustion
See also
Lastest Price from Ammonium chloride manufacturers

US $1.00/g2025-04-21
- CAS:
- 12125-02-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 12125-02-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt