Synthesis of Urea
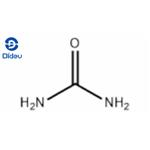
Urea (H2NCONH2) is one of the most important artificial nitrogen fertilisers in the agricultural economy, providing essential nitrogen for plant growth. It is produced by the synthesis of liquid ammonia and gaseous carbon dioxide. In a urea reactor, ammonia and carbon dioxide react to form ammonium carbamate, a portion of which is dehydrated to form urea and water.

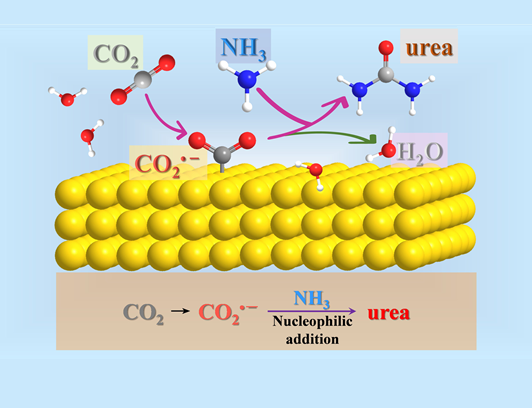
However, the industrial production of urea mentioned above is very energy intensive. Instead, the electrocatalytic method is now considered a promising green method for the synthesis of urea under mild conditions. Urea is synthesised electrochemically under water and ambient conditions. Urea is synthesised by nucleophilic addition during electrochemical CO2 reduction in an electrolyte containing NH4HCO3. The rate of urea production was 0.11 mmol g-1 h-1 at a polycrystalline gold electrode (area: 1 cm2) at a potential of -1.4 V (with respect to a saturated calomel electrode), and the reaction proceeded as follows:
References:
[1] CHUANJU YANG . Electrocatalytic C–N coupling for urea synthesis: a critical review[J]. Green Chemistry, 2024, 26 9. DOI:10.1039/d3gc04920e.You may like
Related articles And Qustion
See also
Lastest Price from Urea manufacturers
US $0.00-0.00/kg2025-12-08
- CAS:
- 57-13-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $280.00-260.00/ton2025-07-15
- CAS:
- 57-13-6
- Min. Order:
- 25ton
- Purity:
- N46
- Supply Ability:
- 5000tons