The Molecular Structure and Properties of Urea
Urea is a vital organic compound used extensively in various industries due to its unique molecular structure and properties. Understanding its structure is key to comprehending its reactivity and widespread applications. In this article, we will explore the structural characteristics of urea and how they contribute to its polar nature, solubility, and reactivity.
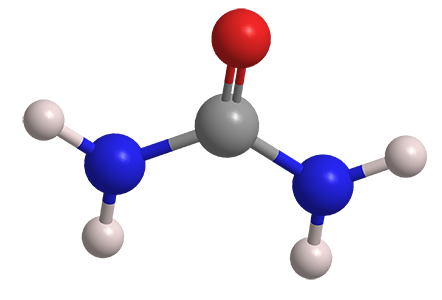
Planar Structure
Urea's molecular structure consists of a carbonyl group and an amino group arranged in a planar hexagonal ring. This arrangement ensures that the carbonyl group and amino group lie on the same plane, resulting in a flat, planar structure.
Polar Molecules
Both the amino group (-NH2) and the carbonyl group (C=O) in the urea molecule are polar functional groups. These polar groups contribute to the polarity of urea as a whole, making it a polar molecule.
Hydrogen Bonding
Urea molecules possess the ability to form hydrogen bonds due to the presence of amino and carbonyl groups. The hydrogen atoms attached to each amino group (-NH3) can form hydrogen bonds with the oxygen atom of the carbonyl group (C=O). These intermolecular hydrogen bonds play a significant role in the properties and applications of urea.
Solubility in Water
The polar nature and hydrogen bonding capabilities of urea make it highly soluble in water. When dissolved in water, urea forms hydrogen bonds with water molecules, creating hydrogen bond networks. This increased solubility is essential for its application in various processes.
Reactivity
The amino and carbonyl groups in the urea molecule exhibit high reactivity. The amino groups can participate in chemical reactions such as acid-base reactions, while the carbonyl group is involved in reactions with aldehydes, ketones, and other compounds. These reactive groups make urea widely utilized in the chemical industry.
Conclusion
Urea's molecular structure yields several critical properties, including its planar arrangement, polarity, the formation of hydrogen bonds, and its enhanced solubility in water. These characteristics not only influence the chemical properties of urea but also drive its extensive applications in various industries. The reactivity of the amino and carbonyl groups expands urea's scope, enabling it to be used in many chemical reactions. Overall, understanding the molecular structure of urea provides insights into its functional properties and diverse utility across different fields.
Related articles And Qustion
See also
Lastest Price from Urea manufacturers
US $0.00-0.00/kg2025-12-08
- CAS:
- 57-13-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $280.00-260.00/ton2025-07-15
- CAS:
- 57-13-6
- Min. Order:
- 25ton
- Purity:
- N46
- Supply Ability:
- 5000tons