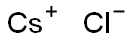Toxicity of cesium chloride
Overview
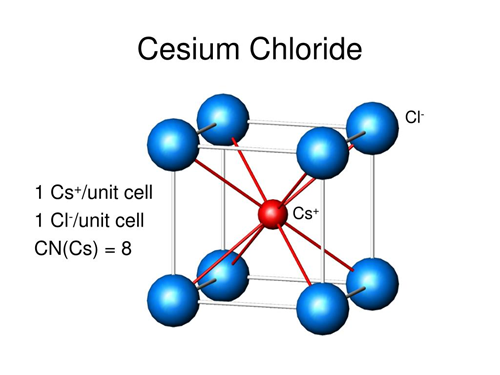
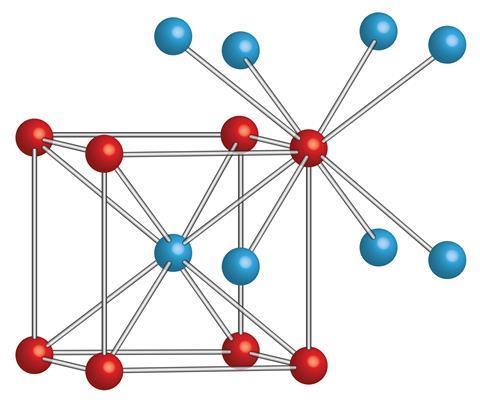
Cesium chloride (Cesium chloride) Cesium chloride is a colorless cubic crystal, which is easily soluble in water, soluble in ethanol and methanol, but insoluble in acetone, and deliquescence in the air. The role of cesium chloride in biological research: density gradient centrifugation is a new centrifugal technology. It can separate molecules with small mass differences. Using a concentrated solution of cesium chloride, with a strong centrifugal force above 105 g, the salt molecules are thrown to the bottom of the centrifuge tube; at the same time, the diffusion effect makes the Cs+ and Cl in the solution dispersed, which is opposite to the direction of the centrifugal force. After centrifugation over time, the solution reaches an equilibrium state[1-2].

Function and mechanism [3]
Cs and K have similar biological characteristics, and can be concentrated in the normal myocardium after injecting blood to make the myocardium visible. At the site of myocardial ischemia or infarction, due to reduced myocardial blood flow or impaired function of myocardial cells, radioactive sparse or defective areas appear. 131Cs can also be concentrated by thyroid cancer cells.
Clinical application [3]
Positive imaging agent for thyroid cancer. 131I or "TeOi-imaging only indicates that the thyroid mass is a "cold nodule". In order to identify its nature, a positive 1Cs thyroid cancer imaging can be done again. If. 1Cs concentration in the "cold nodules" indicates that the possibility of thyroid cancer is very high, and the coincidence rate can reach 70% to 80%; if there is no "1Cs concentration", the possibility of benign lesions is high. This product is also used as myocardial perfusion Imaging Jing, due to the low energy of radiation, is currently not used.
Overview of toxic effects [4]
In the biochemical process, K can be partially replaced, which reduces the K content in muscle cells and red blood cells. Particularly dangerous is the work that converts pure metals into oxides in dry air and into hydroxides in humid air.
For acute poisoning, the rats were given CsOHLDs0=0.76 g/kg (5.70 in rats), CsCl was 2.0, and Cs:SO. It is 2.8, Cs2C03 is 2.3, and CsN03 is 2.39 g/kg. The sensitivity to cesium salt increases in the following order: mice, rats, guinea pigs, rabbits, and cats. R. LDso=3.8, RbOH was 0.84 g/kg (BpyK; XocalⅡ;). Similar to the characteristic neuromuscular device damage seen in hyperkalemia, bradycardia, electrocardiogram changes, and acid-base balance disorders. Autopsy revealed pulmonary bleeding and nutmeg liver.
Chronic poisoning to animals: rats inhaled RbCI and CsCI (30 mg/m3, 4 hours a day, 4 months), and RbOI-I and CsOFI (10 mg/m3, 4 hours a day, 4 months), performance Increased excitability, after the wrong growth, nutritional disorders; hydroxide can also cause upper respiratory stimulation. Autopsy revealed degeneration of kidney and liver cells. Feeding animals with 0.10,4Rb in the food, the offspring born have no living ability.
To people; workers engaged in the production of Rb and Cs (aerosols of mineral dust, metal oxides and their salts in the air) complained: increased stress, fatigue, insomnia, headache, and numbness of the fingers. Objective manifestations, neurasthenia syndrome, combined with vegetative vascular insufficiency, atrial and ventricular conduction disorders, myocardial degeneration; renal stimulation (protein in urine, leaching epithelial cells, hyalinin casts, red blood cells, oxaluria); Chronic inflammation of the upper respiratory tract mucosa; increased incidence of peripheral nervous system (XocHl: a).
Effect on skin and eyes Apply RbCl and CsCl multiple times without causing irritation to rabbit skin and mucous membranes. Application with 4% RbOH and CsOH solution can cause hyperemia, edema, further necrosis and scarring changes. 0.4% of the solution splashed into the eyes can cause the formation of conjunctival ulcers and turbid cornea. Cesium compounds have obvious stimulating effects. There have been cases recorded that when cesium is produced, it can cause allergic dermatitis and occupational eczema.
The maximum allowable concentration is recommended to be 0.3 mg/m3 for rubidium, cesium and their compounds. For cesium hydroxide, it is recommended to be 0.3 mg/m3 (CeKItrle/%110ycTaHoBIIeHHm n and K Bpepan HhlX BeI//, eCTB B B03 and yxe pa60-qe 苡30Hbl approved on June 9-11, 1976). Distribute and excrete C3 and Rb in the body from the blood circulation into the blood in the form of ions. The concentration of Rb in red blood cells is almost three times higher than that in plasma. Rb quickly disappears from the blood and mainly accumulates in the muscles. In the initial stage, the concentration of Cs in muscle, liver, kidney, and spleen is almost equal. Due to redistribution, the main reservoir is muscle, and its concentration is 7-8 times higher than other organs. Cs and Rb are mainly excreted in urine. The half-life of human cs is 70 days.
Production Process
Add 6409 cesium carbonate to 200ral water, and slowly add 37% hydrochloric acid 4009() (AJ 340ml) under stirring. Heating, the reaction released a large amount of carbon dioxide, until the pH was 3, boiled for 0.5h. Then add an appropriate amount of cesium hydroxide to adjust the pH to neutral. Filter to remove residue. The filtrate was concentrated and crystallized. Separate and crystallize at 100. C Dry to get cesium chloride.
References
[1] Cen Fang, Editor-in-Chief, New Curriculum Senior High School Teacher's Manual Biology, Nanjing University Press, 2012.04, p.387
[2] Yang Nanru and Yue Wenhai, editor in chief, Handbook of Atlas of Inorganic Nonmetallic Materials, Wuhan University of Technology Press, 1st edition, November 2000, p.635
[3] Edited by Rui Yaocheng, Handbook of Practical Medicines, People's Military Medical Press, 1st edition, April 2001, p.967
[4] Jin Feng, translated by Zhou Shusen and others, Handbook of Hazardous Substances in Industrial Production Volume 3 (Revised Seventh Edition) Inorganic and Elemental Organic Compounds, Chemical Industry Press, 1st edition, May 1986, page 527
Related articles And Qustion
Lastest Price from Cesium chloride manufacturers

US $100.00/kg2025-04-21
- CAS:
- 7647-17-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $6.00/kg2025-04-21
- CAS:
- 7647-17-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month