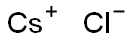General Description of Cesium chloride
Chemical Properties
Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
Structure
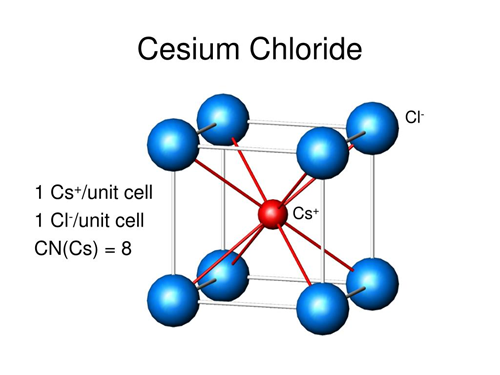
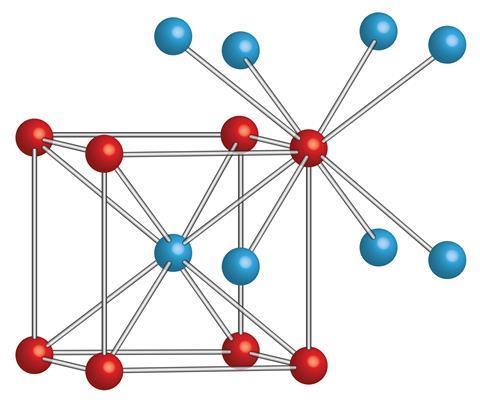
The Cesium chloride structure is composed of interlocking simple cubic lattices of anions and cations. It is the case that in a cubic 1:1 solid where one atom type is much larger than the other that the Cesium chloride type lattice is obtained, it can be thought of as a combination of basketballs and golf balls packed in a cubic manner with the golf balls in the gaps between the basketballs. If the two atom types are similar in size (imagine field hockey balls packed with tennis balls) then in the cubic lattice the structure will be like that of sodium chloride.
Occurrence
Cesium chloride is the inorganic compound with the formula CsCl. This colorless solid is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions. Cesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Cesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
Application
Cesium chloride (CsCl) is an inorganic, colorless, hygroscopic crystalline compound. It has a large mass and is highly soluble in water (1865 g/L). Due to its hygroscopic characteristic, when put in water, it forms a dense solute that is not very viscous. Therefore, it is a good material for equilibrium gradient differential centrifugation where the separation of the particles is size and density dependent. The method is used to separate cellular compartments (organelles), different types of DNA (viral, plasmid, mitochondrial, etc.), or RNA types (mRNA, tRNA, and rRNA).
Caesium chloride is widely used medicine structure in isopycnic centrifugation for separating various types of DNA. It is a reagent in analytical chemistry, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine applications such as treatment of cancer and diagnosis of myocardial infarction. Another form of cancer treatment was studied using conventional non-radioactive CsCl. Whereas conventional caesium chloride has a rather low toxicity to humans and animals, the radioactive form easily contaminates the environment due to the high solubility of CsCl in water. Spread of 137CsCl powder from a 93-gram container in 1987 in Goiânia, Brazil, resulted in one of the worst-ever radiation spill accidents killing four and directly affecting 249 people.
Cesium chloride (CsCl), once a standard centrifugation medium for both analytical and preparative separation of nucleic acids, enjoys very limited use today. Only ultrapure nuclease-free preparations of CsCl should be purchased because CsCl has only a limited ability to inhibit RNase activity. If necessary, impure, solid CsCl may be baked at 200° for 4–6 h to remove residual RNase activity prior to exposure to RNA. The use of CsCl as a menstruum for the purification of nucleic acids in general has declined significantly in favor of newer resins and silica filters, the use of either of which precludes the requirement for density gradient (isopycnic) centrifugation.
Additionally, Cesium chloride (CsCl) is a mineral salt that is sometimes taken either by mouth, or by injection into the body, by cancer patients who seek alternative treatments. However, no CsCl products have been approved by FDA to treat cancer or other diseases.
Toxicity
Caesium chloride has a low toxicity to humans and animals. Its median lethal dose (LD50) in mice is 2300 mg per kilogram of body weight for oral administration and 910 mg/kg for intravenous injection. The mild toxicity of CsCl is related to its ability to lower the concentration of potassium in the body and partly substitute it in biochemical processes. When taken in large quantities, however, can cause a significant imbalance in potassium and lead to hypokalemia, arrythmia, and acute cardiac arrest. However, caesium chloride powder can irritate the mucous membranes and cause asthma.
You may like
Related articles And Qustion
Lastest Price from Cesium chloride manufacturers

US $100.00/kg2025-04-21
- CAS:
- 7647-17-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $6.00/kg2025-04-21
- CAS:
- 7647-17-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month