What is N,N-Dimethylaniline?
Aug 20,2020
Introduction
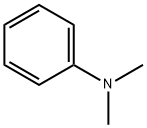
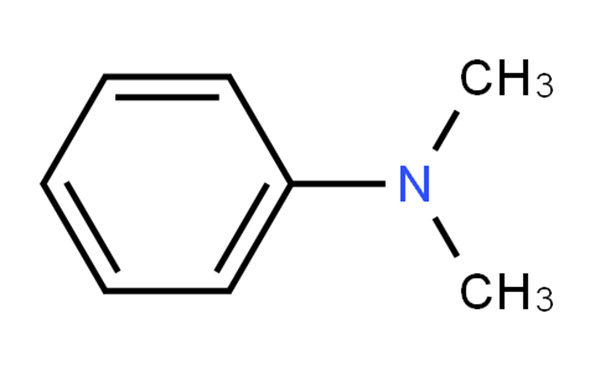
N,N-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It consists of a tertiary amine, featuring dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet.
N,N-Dimethylaniline is used in production of dyestuffs, as a solvent, a reagent in methylation reactions, and a hardener in fiberglass reinforced resins.
Chemical Properties
Dimethylaniline is a yellow to brown colored oily liquid with a fishlike odor. Less dense than water and insoluble in water. Freely soluble in alcohol, acetone, benzene, chloroform, diethyl ether, ethanol and acid solution. Stable under ordinary conditions of use and storage. Combustible. Dimethylaniline is a weak base incompatible with strong oxidizing agents, strong acids, acid chlorides, acid anhydrides, chloroformates, halogens. It emits toxic fumes of nitriogen oxides, carbon oxides, and aniline when heated to decomposition.
Toxic by ingestion, inhalation, and skin absorption.
Preparation and reactions
DMA was first reported in 1850 by the German chemist A. W. Hofmann, who prepared it by heating aniline and iodomethane:
C6H5NH2 + 2 CH3I → C6H5N(CH3)2 + 2 HI
DMA is produced industrially by alkylation of aniline with methanol in the presence of an acid catalyst:
C6H5NH2 + 2 CH3OH → C6H5N(CH3)2 + 2 H2O
Similarly, it is also prepared using dimethyl ether as the methylating agent.
Dimethylaniline undergoes many of the reactions expected for an aniline, being weakly basic and reactive toward electrophiles. For example, it is nitrated to produce tetryl, a derivative with four nitro groups which was once used as explosive. It is lithiated with butyllithium. Methylating agents attack the amine to give the quaternary ammonium salt:
C6H5N(CH3)2 + (CH3O)2SO2 → C6H5N(CH3)3CH3OSO3
Applications
N,N-Dimethylaniline is used as chemical intermediate in the manufacturing of vanillin, Michler's ketone, and dyes such as Acid Red 2, Basic Green 4 and Basic Violet 1. It is also used as a solvent and an activator for polyesters and as an alkylating agent. It is used as an acid scavenger or accepter in the manufacture of beta-lactam antibiotics such as penicillin and cephalosporin.
N,N-Dimethylaniline serves as a promoter in the curing of polyester and vinyl ester resins. This compound can be used on its own with benzoyl peroxide (BPO) type catalysts or in combination with cobalt 6% promoters with methyl ethyl ketone (MEKP) type catalysts. These systems give rapid cure at room temperature. DMA helps the catalyst to start the chemical reaction. Promoters must never be mixed directly with catalyst since a violent explosive reaction results.

DMA is a key precursor to commercially important triarylmethane dyes such as malachite green and crystal violet. DMA serves as a promoter in the curing of polyester and vinyl ester resins. DMA is also used as a precursor to other organic compounds. A study of the in vitro metabolism of N,N-dimethylaniline using guinea pig and rabbit preparations and GLC techniques has confirmed N-demethylation and N-oxidation as metabolic pathways, and has also established ring hydroxylation as a metabolic route. Its derivative 2,4-dimethylaniline is a recalcitrant degradant of the pesticide amitraz and is also an industrial pollutant which is genotoxic, teratogenic and carcinogenic.
Stablilisers
DMA is used as a stabilizer for colorimetric peroxidase determination.
Pharma
DMA is used to manufacture cephalosporin V, madribon, sulphormethoxine and flucytosine in medical industry. It is used as an acid scavenger in the synthesis of penicillins and cephalosporins and has been reported as a contaminant of commercial preparations of those antibiotics at levels of up to 1500 ppm.
Health Hazard
Clinical signs of intoxication with N,N-dimethylaniline in man are headaches, cyanosis, dizziness, labored breathing, paralysis and convulsions (HSDB 1989). It is absorbed through the skin to produce a dangerous methemoglobinemia (Gosselin 1984). Treatment is similar to that of aniline with the object of managing methemoglobinemia.
Suspected carcinogen with equivocal tumorigenic data. Human poison by ingestion. Moderately toxic by inhalation and skin contact. A skin irritant. Human systemic effects by ingestion: nausea or vomiting. Physiological action is similar to, but less toxic than, adne. A central nervous system depressant. Mutation data reported. Flammable liquid when exposed to heat, flame, or oxidizers.
Explodes on contact with benzoyl peroxide or disopropyl peroxydicarbonate. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits htghly toxic fumes of adne and NOx.
Carcinogenicity
A 2-year corn oil gavage bioassay conducted by NTP in F344/N rats (0.3 or 30 mg/kg) and B6C3F1 mice (0, 15, or 30 mg/kg) for 103 weeks concluded that there was some evidence of carcinogenic activity for male F344/N rats as indicated by the increased incidences of sarcomas or osteosarcomas in the spleen; there was no evidence of carcinogenic activity in the female rats or male mice; there was equivocal evidence of carcinogenic activity for female mice as indicated by an increased incidence of squamous cell papillomas of the forestomach. Both rats and mice could have tolerated doses higher than those used in these studies.
You may like
What is Posaconazole?
Mar 29, 2022
Paracetamol Uses, Overdosage & toxicity
Feb 23, 2022
What is Diclofenac sodium?
Feb 11, 2020
Related articles And Qustion
See also
Lastest Price from N,N-Dimethylaniline manufacturers
N,N-Dimethylaniline

US $0.00/kg2025-05-16
- CAS:
- 121-69-7
- Min. Order:
- 1000kg
- Purity:
- 99
- Supply Ability:
- 20mt
N,N-Dimethylaniline

US $0.00/KG2025-04-21
- CAS:
- 121-69-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt