Paracetamol Uses, Overdosage & toxicity
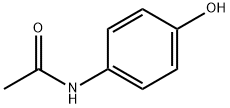
Paracetamol (acetaminophen) was first used in 1893 and is the only remaining p-aminophenol available in clinical practice. I t is the active metabolite of the earlier, more toxic drugs acetanilide and phenacetin. I ts structure is shown in Fig. 1. Paracetamol is an effective analgesic and antipyretic but has no anti-inflammatory activity. I n recommended doses, it is safe and has remarkably few adverse effects.
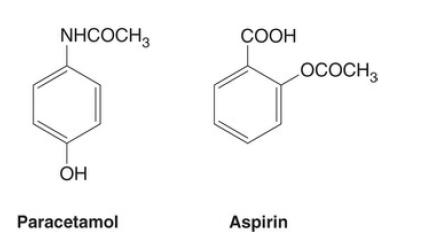
FIG. 1 The structures of paracetamol and aspirin.
Mechanism of action
The mechanism of action of paracetamol is not well understood, but it may
act in a similar fashion to NSAIDs, with inhibition of cyclo-oxygenase
enzymes COX-1 and COX-2 (see later) to reduce the phenoxyl radical
formation required for COX-1 and 2 activity and prostaglandin synthesis. I t
has selectivity for inhibition of prostaglandin synthesis with low
concentrations of peroxidases and arachidonic acid, but limited effect at
higher concentrations and, therefore, has limited anti-inflammatory effects.
Unlike opioids, paracetamol has no well-defined endogenous binding sites.
In some circumstances, it may exhibit a preferential effect on COX-2
inhibition. There is growing evidence of a central antinociceptive effect of
paracetamol. It has also been found to prevent prostaglandin production at
the cellular transcriptional concentration, independent of COX activity.
Pharmacokinetics
Paracetamol is absorbed rapidly from the small intestine after oral
administration; peak plasma concentrations are reached after 30–60min. It
may also be given rectally and intravenously (either as paracetamol or the
prodrug propacetamol). It has good oral bioavailability (70%–90%); rectal absorption is more variable (bioavailability ~50%–80%) with a longer time to
reach peak plasma concentration. The plasma half-life is approximately 2–3h.
Paracetamol is metabolised by hepatic microsomal enzymes mainly to the glucuronide, sulphate and cysteine conjugates. N one of these metabolites is pharmacologically active. A minimal amount of the metabolite N-acetyl-pamino- benzoquinone imine is normally produced by cytochrome P450– mediated hydroxylation. This reactive toxic metabolite is rendered harmless by conjugation with liver glutathione, then excreted renally as mercapturic derivatives. With larger doses of paracetamol, the rate of formation of the reactive metabolite exceeds that of glutathione conjugation, and the reactive metabolite combines with hepatocellular macromolecules, resulting in cell death and potentially fatal hepatic failure. The formation of this metabolite is increased by drugs inducing cytochrome P450 enzymes, such as barbiturates or carbamazepine.
Pharmacodynamics
Paracetamol is effective in both acute and chronic pain and is available for oral or intravenous use. I t is an effective postoperative analgesic but probably less effective than NSAIDs in many situations. I t may reduce postoperative opioid requirements by up to 30%. The combination of paracetamol with an NSAID also improves efficacy. Paracetamol is also a very effective antipyretic, a centrally mediated effect.
Overdose and hepatic toxicity
In overdose there is the potential for the toxic metabolite described earlier to cause centrilobular hepatocellular necrosis, occasionally with acute renal tubular necrosis. The threshold dose in adults is approximately 10–15g. A ccidental overdosage can occur if combined preparations such as cocodamol are used together with paracetamol. Doses of more than 150mgkg-1taken within 24 h may result in severe liver damage, hypoglycaemia and acute tubular necrosis. Individuals taking enzyme-inducing agents are more likely to develop hepatotoxicity. More recently there has been debate about lowering the standard recommended dose of 4g daily (or 1gqds) for safety reasons, particularly in frail or elderly patients.
Early signs include nausea and vomiting, followed by right subcostal pain and tenderness. Hepatic damage is maximal 3–4 days after ingestion and may lead to liver failure and death. Treatment consists of gastric emptying and the specific antidotes methionine and acetylcysteine. The former offers effective protection up to 10–12h after ingestion. A cetylcysteine is effective within 24h and perhaps beyond. The plasma paracetamol concentration related to time from ingestion indicates the risk of liver damage. N-acetylcysteine is given if the plasma paracetamol concentration is more than 200 mg L–1 at 4 h and 6.25 mg L–1 at 24 h after ingestion.
You may like
Related articles And Qustion
See also
Lastest Price from Acetaminophen manufacturers

US $0.00-0.00/kg2025-07-11
- CAS:
- 103-90-2
- Min. Order:
- 1kg
- Purity:
- 99%pure
- Supply Ability:
- 20 tons

US $50.00/KG2025-06-27
- CAS:
- 103-90-2
- Min. Order:
- 1KG
- Purity:
- 99.%
- Supply Ability:
- 10 ton