What is Baloxavir marboxil?Pharmacodynamics,Medical uses,Resistance
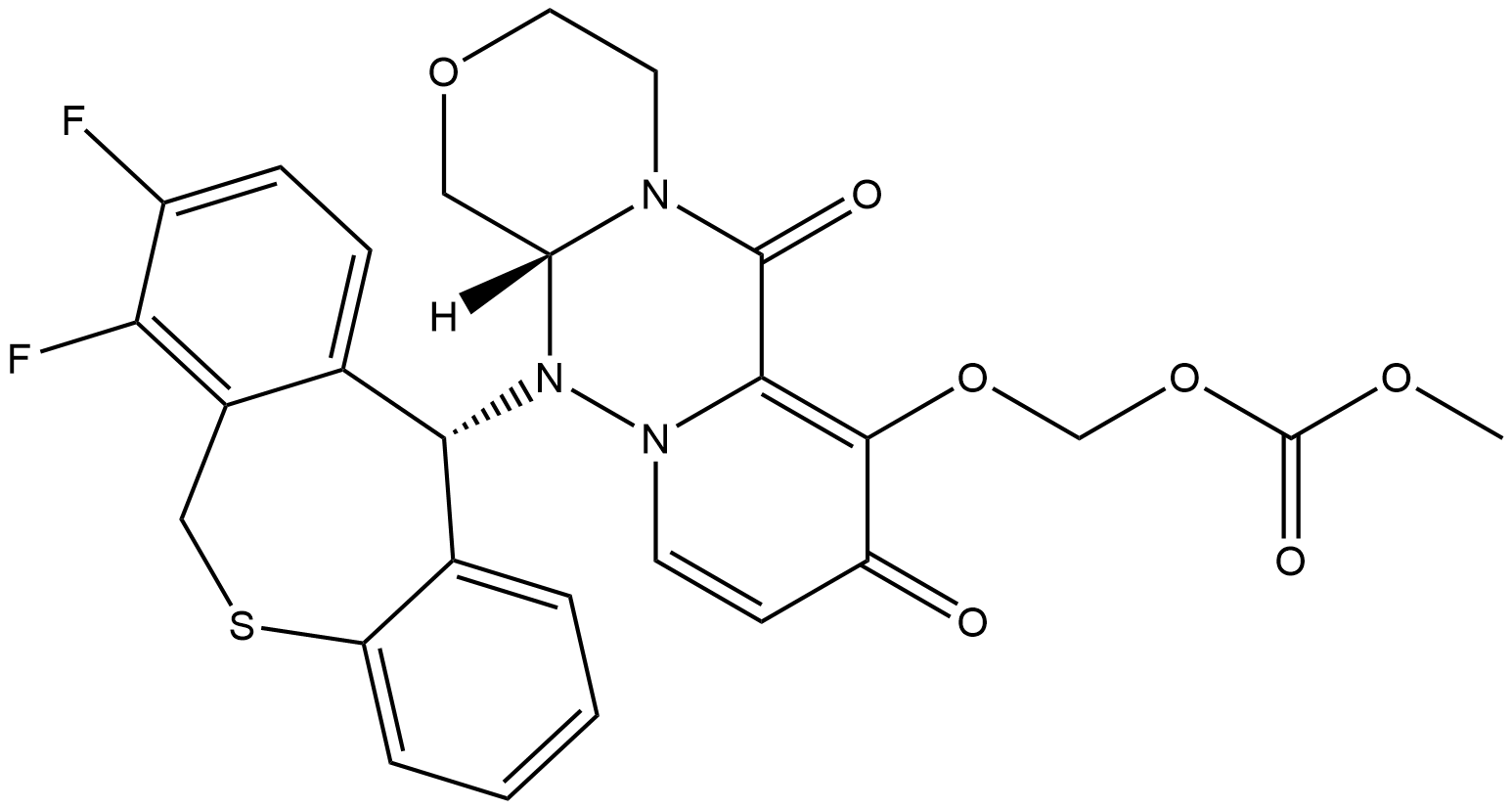
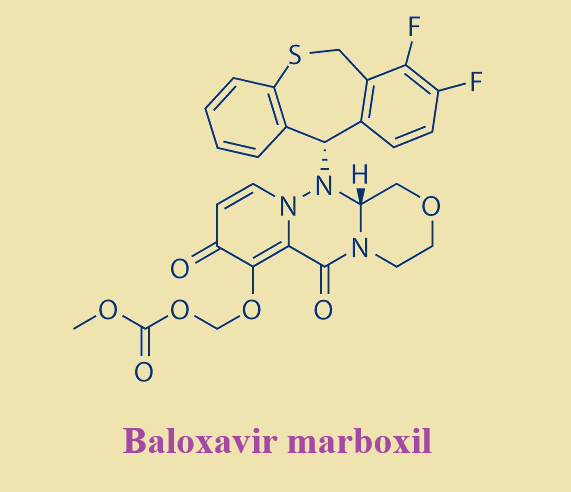
Baloxavir marboxil is an antiviral drug developed by Shionogi Co., a Japanese pharmaceutical company and Roche for the treatment of influenza A and influenza B infections. The drug was initially approved for use in Japan in February 2018 and approved by the FDA on October 24, 2018 for the treatment of acute uncomplicated influenza in patients 12 years of age and older who have been symptomatic for no more than 48 hours Label. Baloxavir marboxil, a cap-endonuclease inhibitor, has a unique mechanism of action when compared to the currently existing neuraminidase inhibitor drug class used to treat influenza infections.

Pharmacodynamics
Baloxavir marboxil is a selective inhibitor of influenza cap-dependent endonuclease which prevents polymerase function and therefore influenza virus mRNA replication 5, 3. It has shown therapeutic activity against influenza A and B virus infections, including strains resistant to current antiviral agents 1. This drug inhibits an enzyme required for viral replication, thus rapidly treating flu virus infection 5, Label and alleviating the symptoms associated with infection. A single dose of this agent was shown to be superior to placebo in relieving influenza symptoms and superior to both oseltamivir and placebo drug in virologic outcomes (marked by decreased viral load)
Medical uses
Baloxavir marboxil is an influenza medication, an antiviral,that is taken as a single dose tablet,by mouth, by individuals that are 12 years of age or older,that have presented symptoms of this infection for no more than 48 hours.The efficacy of baloxavir marboxil administered after 48 hours has not been tested.
Metabolism
Baloxavir marboxil is a prodrug that is converted by hydrolysis to baloxavir, the active form that exerts anti-influenza virus activity Label.
Resistance
In 2.2% of baloxavir recipients in the Phase II trial and in about 10% of baloxavir recipients in the Phase III trial, the infecting influenza strain had acquired resistance to the drug, due to variants of the polymerase protein displaying substitutions of isoleucine-38, specifically, the I38T, I38M, or I38F mutations.There is continuing research into and clinical concern over the resistance appearing in patients, in response to treatment with this drug.
Related articles And Qustion
See also
Lastest Price from Baloxavir marboxil manufacturers

US $0.00/kg2025-06-20
- CAS:
- 1985606-14-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-05-04
- CAS:
- 1985606-14-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton