Introduction to the synthesis method of Baloxavir marboxil
Description
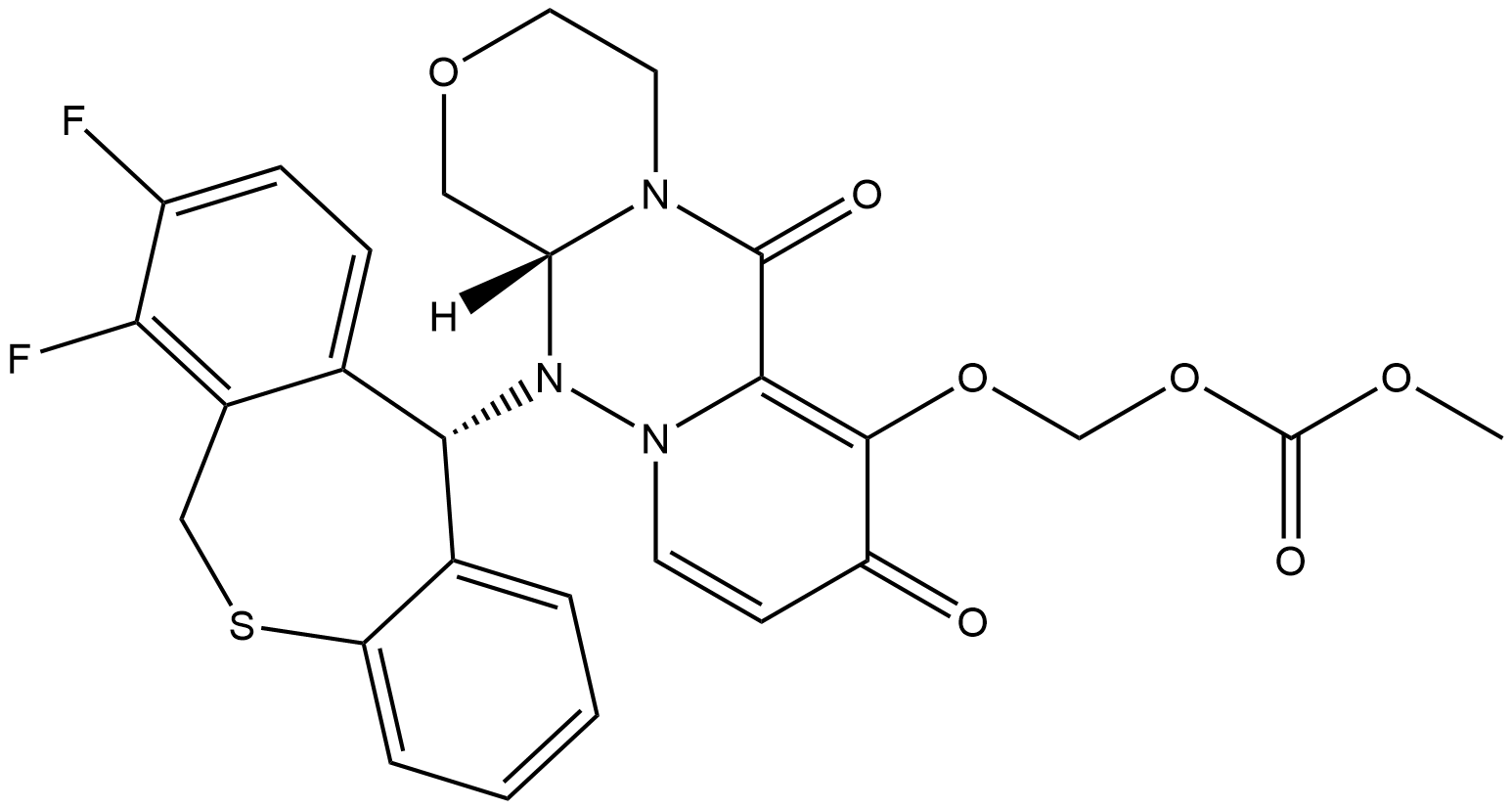
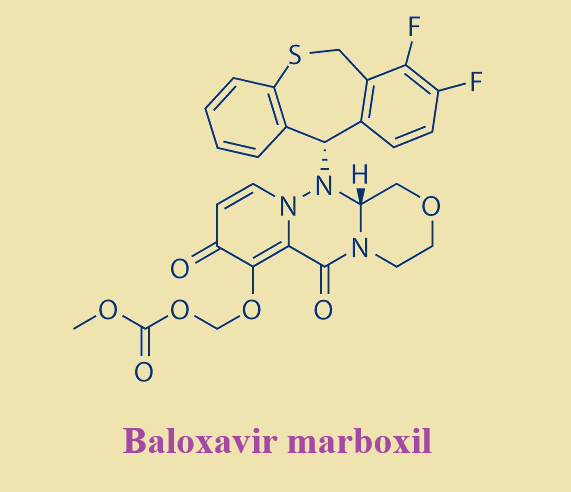
Baloxavir marboxil, the prodrug of baloxavir acid, is a first-in-class, small molecule inhibitor of the polymerase acidic (PA) protein subunit of the influenza virus polymerase complex. Baloxavir is a novel cap-dependent endonuclease inhibitor that blocks influenza virus proliferation by inhibiting the initiation of mRNA synthesis. This is in contrast to neuraminidase inhibitors, which impair viral release from infected host cells[1].
Uses and history
Baloxavir marboxil was discovered by Shionogi, who licensed their rights to Roche in February 2016 for development and commercialization except in Taiwan and Japan (Shionogi maintained its rights in these two countries). In 2018, baloxavir marboxil received its first approval from the Pharmaceuticals and Medical Devices Agency of Japan (PMDA) for treating influenza A or B virus infections. Later the same year, the drug was also approved by the USFDA for the treatment of acute uncomplicated influenza (flu) in patients 12 years of age and older who have been symptomatic for no more than 48 hours.
Synthetic method
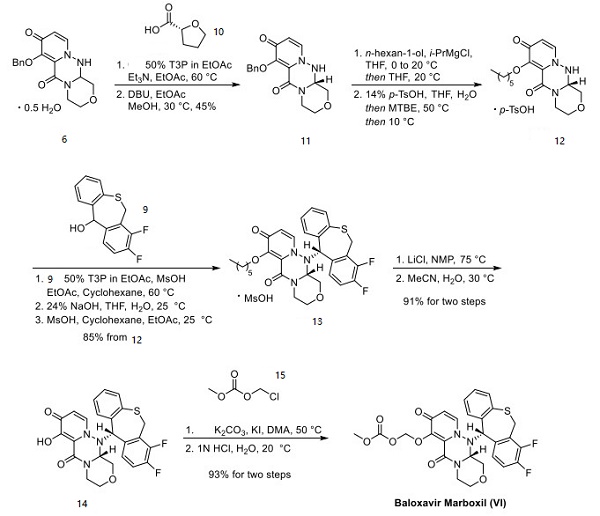
Shionogi has reported two unique synthetic approaches to baloxavir, each originating from different starting materials[2]. This approach strategically hinges upon the union of a piperazine tricyclic core and a tricyclic diaryl mercaptan. Acid 1 was methylated and then subjected to Boc-hydrazine under weakly acidic conditions to furnish ester 2, which reacted with amine 5 to afford racemic hemihydrate 6. Amine 5 can be prepared by the alkylation of phthalimidyl alcohol 3 with bromide 4, followed by hydrazine-mediated phthalimide cleavage.
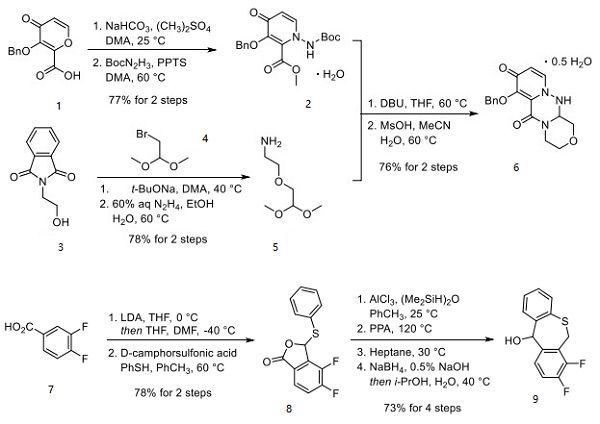
Separately, benzothiepine 9 was constructed as outlined in above. Benzoic acid 7 was o-lithiated prior to quenching with DMF. The lactone in 8 was formed upon acidification with D-CSA. Intramolecular Friedel−Crafts followed by acidification and reduction furnished benzothiepine alcohol 9 in 73% yield. The final steps in the assembly of baloxavir marboxil are described as follows. Enantiomeric resolution of 6 was performed by reacting 6 with commercial chiral acid 10 followed by recrystallization from warm ethyl acetate. The diastereomer corresponding to the desired geometry was then collected and treated with DBU, which provided an enantiomerically enriched free base 11. Although this reaction was exemplified on a kilogram scale, the chiral purity was not reported. The benzyl ether within 11 was then converted to the corresponding n-hexyl ether using n-hexanol and isopropyl magnesium chloride, making way for the isolation of tosylate salt 12 after treatment with p-TsOH. Next, 9 was subjected to propylphosphonic anhydride (T3P) under acidic conditions and coupled with fragment 12 to form the core structure of baloxavir marboxil. Subsequent treatment with base and methanesulfonic acid led to the formation of mesylate salt 13. Removal of the n-hexyl ether was facilitated by lithium chloride in warm NMP, followed by isolation of phenol 14 upon precipitation from a warm acetonitrile/water mixture. Subsequent alkylation with alkyl chloride 15 preceded careful treatment with acid to furnish baloxavir marboxil.
References
[1] Shirley, Matt. “Baloxavir Marboxil: A Review in Acute Uncomplicated Influenza.” Drugs (2020): 1109–1118.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
Related articles And Qustion
See also
Lastest Price from Baloxavir marboxil manufacturers

US $0.00/kg2025-06-20
- CAS:
- 1985606-14-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-05-04
- CAS:
- 1985606-14-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton