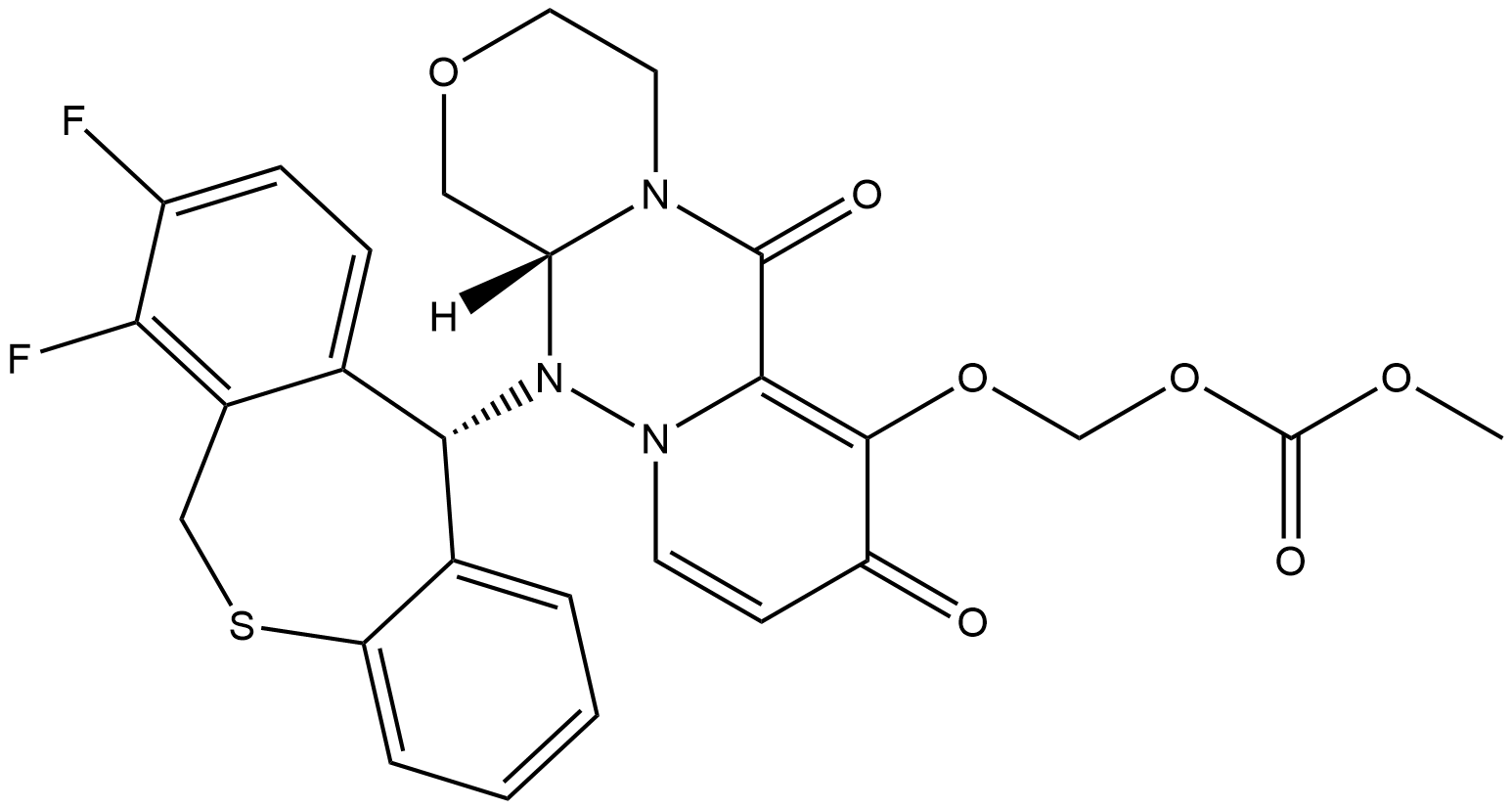
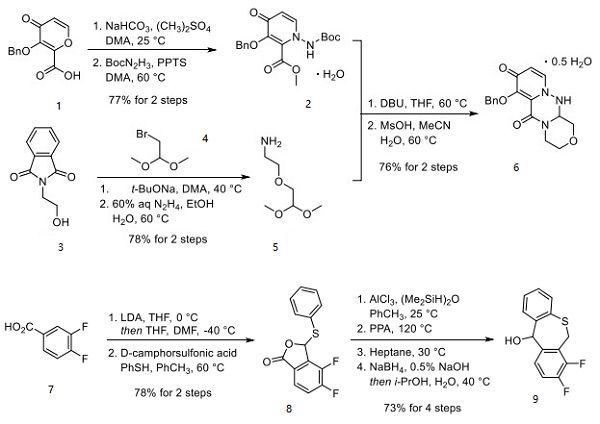
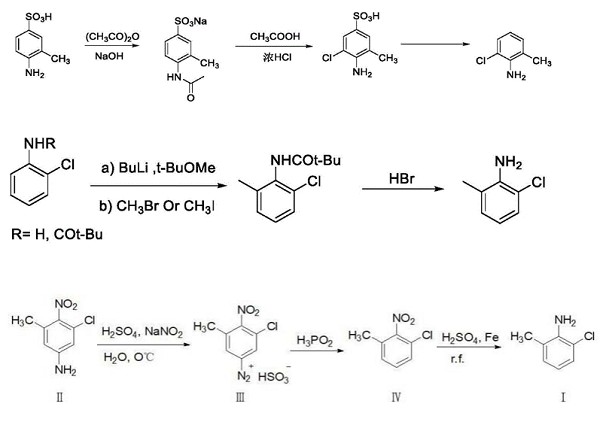
Is baloxavir marboxil safe for oral administration?
Yes. Baloxavir marboxil is well tolerated as a single oral dose, has a favorable safety profile, and has a favorable pharmacokinetic profile, including a long half-life (49-91 hours), supporting single oral administration. Baloxavir is approved in the US for the treatment of acute uncomplicated influenza in patients aged ≥12 years with symptoms lasting ≤48 hours, as a single oral dose.
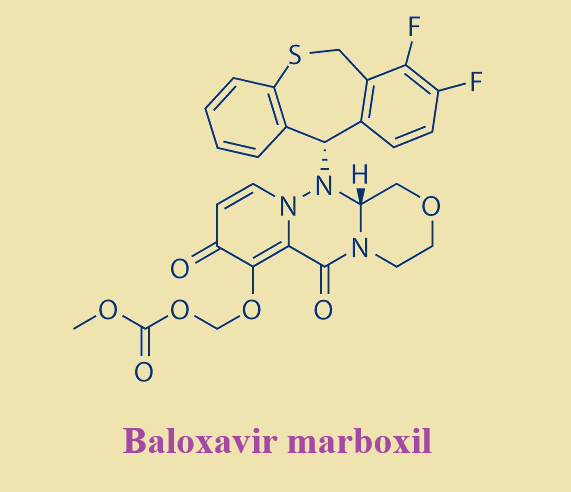
Baloxavir marboxil is a new drug developed by Shionogi, Japan, for the treatment of seasonal influenza infection. Baloxavir marboxil is a prodrug that is metabolized to baloxavir acid and inhibits viral replication by inhibiting the cap-dependent endonuclease. The drug is available in Japan, the US, and Europe. Baloxavir marboxil is well tolerated, with few treatment-emergent adverse events, with diarrhea, bronchitis, nausea, nasopharyngitis, and headache occurring in more than 1% of patients, and no serious adverse events/deaths. Baloxavir marboxil is sensitive to both influenza A and B viruses, although influenza B strains are more resistant due to changes in amino acid residues at the binding site.
In a randomized, double-blind, placebo- and oseltamivir-controlled Phase III trial, data showed that baloxavir marboxil was effective in improving influenza symptoms in otherwise healthy adolescents and adults and those at high risk for influenza complications, with efficacy similar to oseltamivir. In addition, there is evidence that baloxavir marboxil can reduce influenza viral load faster than oseltamivir.
It has the advantage of a single oral dose and provides a useful alternative to neuraminidase inhibitors for the treatment of acute uncomplicated influenza in adolescents and adults.
References:
[1] HIROKI KOSHIMICHI. Safety, Tolerability, and Pharmacokinetics of the Novel Anti-influenza Agent Baloxavir Marboxil in Healthy Adults: Phase I Study Findings.[J]. Clinical Drug Investigation, 2018, 38 12. DOI:10.1007/s40261-018-0710-9.[2] DUFRASNE F. Baloxavir Marboxil: An Original New Drug against Influenza.[J]. ACS Applied Energy Materials, 2021. DOI:10.3390/ph15010028.
[3] SHIRLEY M. Baloxavir Marboxil: A Review in Acute Uncomplicated Influenza.[J]. ACS Chemical Neuroscience, 2020. DOI:10.1007/s40265-020-01350-8.
You may like
Related articles And Qustion
See also
Lastest Price from Baloxavir marboxil manufacturers

US $0.00/kg2025-06-20
- CAS:
- 1985606-14-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-05-04
- CAS:
- 1985606-14-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton